Abstract
Seasonal activity and environmental and hormonal regulation of development and diapause were studied in three different orders of tropical insects at Barro Colorado Island (9°N), Panama.
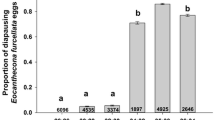
The seed bug, Jadera aeola, reproduces in the late dry season coincident with mass production of its food, seeds of Sapindaceae. Newly emerged adults enter diapause. Population density and food, but not photoperiod, appear to be involved in diapause regulation. Juvenile hormone analogue (JHA) stimulates sexual maturation in both sexes.
The fungus beetle, Stenotarsus rotundus, aggregates at the base of a palm tree where it remains in imaginai diapause for up to 10 months. In addition to daylength (Tanaka et al., 1987), humidity plays an important role in regulating diapause termination. Gonad and flight muscle development occur more rapidly at high humidity than at low humidity. Beetles treated with JHA initiate oocyte and flight muscle development but not testis development. 20-hydroxyecdysone has no effect on diapause termination.
Flesh flies (Sarcophagidae), when monitored with a meat-baited trap, show striking seasonal fluctuations in abundance. Yet, we found no evidence for diapause in three fly species reared in the laboratory under a range of photoperiod and temperature conditions.
Résumé
L’activité àu cours de l’année et la régulation du développement et de la diapause de trois ordres d’insectes tropicaux ont été étudiées dans l’île de Barro Colorado, Panama, 9°N.
L’hemiptère Jadera aeola, se réproduit dans la dernière moitié de la saison sèche, au màme temps de la production massive de sa nourriture, les fruits des Sapindaceae. Les adultes jusqu’après l’éclosion, entrent dans une diapause. La densité de la population et la présence de la nourriture, mais pas la photopériode, paraissent àtre impliquées dans la régulation de la diapause. Un analogue de l’hormone juvénile (JHA) stimule la maturité sexuelle des deux sexes.
Le coleoptère Stenotarsus rotundus se rassemble au base d’un palmier où l’agrégation séjourne, en diapause adulte, durant presque dix mois. A part de la photopériode (Tanaka et al., 1987), l’humidité joue un rôle important dans la régulation de la conclusion de la diapause. Le développement des gonades et des muscles du vol est plus vite dans une humidité élevée que dans une humidité basse. Les coleoptères traités avec JHA commencent le développement des oöcytes et des muscles du vol, mais pas des testes. La hydroxyecdysone n’a aucun effet sur la terminaison de la diapause.
Les mouches carnassières (Sarcophagidae), étudiées avec une piège amorcée de la foie de poule, montrent des fluctuations marquées en abondance saisonelles. Cependant, nous n’avons trouvé aucune indication d’une diapause dans les trois espèces de mouches élevées dans le laboratoire sous des conditions diverses de photopériode et de température.
Similar content being viewed by others
References
Ankersmit G. W. and Adkisson P. L. (1967) Photoperiodic responses of certain geographical strains of Pectinophora gossypiella (Lepidoptera). J. Insect Physiol. 13, 553–564.
Borror D. J., Delong D. M. and Triplehorn C. A. (1976) An Introduction to the Study of Insects, 4th edn. Hall, Rinehart and Wilson, New York.
Carlisle D. B., Ellis P. E. and Betts E. (1965) The influence of aromatic shrubs on sexual maturation in the desert locust Schistocerca gregaria. J. Insect Physiol. 11, 1541–1558.
Croat T. B. (1978) Flora of Barro Colorado Island. Stanford Univ. Press, Stanford.
Denlinger D. L. (1971) Embryonic determination of pupal diapause in the flesh fly Sarcophaga crassipalpis. J. Insect Physiol. 17, 1815–1822.
Denlinger D. L. (1972) Induction and termination of pupal diapause in Sarcophaga (Diptera: Sarcophagidae). Biol. Bull. 142, 11–24.
Denlinger D. L. (1979) Pupal diapause in tropical flesh flies: environmental and endocrine regulation, metabolic rate and genetic selection. Biol. Bull. 156, 31–46.
Denlinger D. L. (1985) Hormonal control of diapause. In Comprehensive Insect Physiology, Biochemistry and Pharmacology. (Edited by Kerkut G. A. and Gilbert L. I.), pp. 353–412. Pergamon Press, Oxford.
Denlinger D. L. (1986) Dormancy in tropical insects. Ann. Rev. Ent. 31, 239–264.
Denlinger D. L. and Shukla M. (1984) Increased length and variability of the life cycle in tropical flesh flies (Diptera: Sarcophagidae) that lack diapause. Ann. ent. Soc. Am. 77, 46–49.
Dingle H. and Arora G. (1973) Experimental studies of migration in bugs of the genus Dysdercus. Oecologia 12, 119–140.
Dodge H. R. (1968) The Sarcophagidae of Barro Colorado Island, Panama (Diptera). Ann. Ent. Soc. Am. 61, 421–450.
Janzen D. H. (1983) Costa Rican Natural History. Chicago Univ. Press, Chicago.
Henrich V. C. and Denlinger D. L. (1982) A maternal effect that eliminates pupal diapause in progeny of the flesh fly, Sarcophaga bullala. J. Insect Physiol. 28, 881–884.
Milton K. (1982) Dietary quality and population regulation in a howler monkey population. In The Ecology of a Tropical Forest (Edited by Leigh E. G., Rand A. S. and Windsor D. M.), pp. 273–289. Smithsonian Institution Press, Washington D.C.
Saunders D. S. (1982) Insect Clocks, 2nd edn. Pergamon Press, Oxford.
Scheites P. (1978) The condition of the host plant during aestivation-diapause of the stalk borers Chilo partellus and Chilo orichalcociliella (Lepidoptera, Pyralidae) in Kenya. Ent. Exp. Appl. 24, 479–488.
Smythe N. (1982) The seasonal abundance of night flying insects in a neotropical forest. In The Ecology of a Tropical Forest (Edited by Leigh E. G., Rand A. S. and Windsor D. M.), pp. 309–318.
Tanaka S. (1986) Sexual dimorphism in Stenotarsus rotundus. Coleop. Bull. 40, 45–47.
Tanaka S. and Wolda H. (1988) Oviposition behavior and diel rhythms of flight and reproduction in two species of tropical seed bugs. Proc. Kon. Nederl. Akad. Wetensch. Ser. C. (in press).
Tanaka S., Denlinger D. L. and Wolda H. (1987) Daylength and humidity as environmental cues for diapause termination in a tropical beetle. Physiol. Ent. 12, 213–224.
Tanaka S., Wolda H. and Denlinger D. L. (1988) Abstinence from mating by sexually mature males of the fungus beetle, Stenotarsus rotundus, during a tropical dry season. Biol tropica (in press).
Tauber M. J., Tauber C. A. and Masaki S. (1986) Seasonal Adaptation of Insects. Oxford Univ. Press, Oxford.
Wolda H. (1983) Spatial and temporal variation in abundance in tropical animals. In The Tropical Rainforest: Ecology and Management (Edited by Sutton S. L., Whitmore T. C. and Chadwick A. C.), pp. 93–105. Blackwell Scientific Publ., Oxford.
Wolda H. and Denlinger D. L. (1984) Diapause in a large aggregation of a tropical beetle. Ecol. Ent. 9, 217–230.
Usua E. J. (1970) Diapause in the maize stemborer. J. Econ. Ent. 63, 1605–1610.
Usua E. J. (1973) Induction of diapause in the maize stemborer, Busseola fusca. Ent. Exp. Appl. 16, 322–328.
Young A. M. (1982) Population Biology of Tropical Insects. Plenum Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanaka, S., Wolda, H. & Denlinger, D.L. Seasonality and its Physiological Regulation in Three Neotropical Insect Taxa from Barro Colorado Island, Panama. Int J Trop Insect Sci 8, 507–514 (1987). https://doi.org/10.1017/S1742758400022554
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1017/S1742758400022554