Abstract
Background
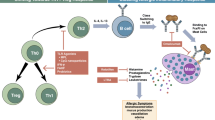
Food allergy (FA) is a worldwide health problem, affecting nearly 10% of all populations, with no prophylactic options or regulatory treatment available until now. Fisetin, a biologically active flavonoid, and telmisartan, the highly selective competitive AT1 receptor antagonist, recently exhibited potent anti-inflammatory and immunomodulatory activities. In the present study, we have evaluated the possible anti-inflammatory and immunomodulatory activities of fisetin and telmisartan each alone or in low-dose combination in a mouse model of FA.
Methods
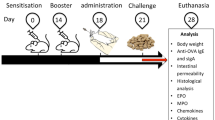
For induction of FA, eight-week-old BALB/c mice, sensitized by two ip injection of 50 μg ovalbumin (OVA) and 1 mg alum at day 0 and 7. Then, each mouse challenged with 10 mg OVA at days 14, 16, 18, and 21. On the 28th day, the fifth challenge carried out by oral administration of 50 mg OVA. Either fisetin (1 or 3 mg/kg/d), telmisartan (1 or 3 mg/kg/d) or a combination of fisetin 1 mg/kg/d and telmisartan 1 mg/kg/d received orally from the 13th day till 28th day. In challenge days, the treatments received one-hour before the challenge.
Results
Our data showed that fisetin and telmisartan each alone or in low-dose combination attenuated the anaphylactic manifestation, decreased blood eosinophilic count, serum OVA-specific IgE, and IL-4 levels, the intestinal total and degranulated mast cells count, and CD4+ immunohistochemical expression. Furthermore, they enhanced the serum IFN-γ level and abrogated the intestinal histopathological changes induced by OVA in mice.
Conclusion
Either fisetin, telmisartan or their low-dose combination could be promising in the management of FA.
Similar content being viewed by others
References
Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018;141:41–58.
Muehling LM, Lawrence MG, Woodfolk JA. Pathogenic CD4+ T cells in patients with asthma. J Allergy Clin Immunol 2017;140:1523–40.
Sampson HA, O’mahony L, Burks AW, Plaut M, Lack G, Akdis CA. Mechanisms of food allergy. J Allergy Clin Immunol 2018;141:11–9.
Rivas MN, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol 2016;138(801–11):e9.
Dagenais NJ, Jamali F. Protective effects of angiotensin II interruption: evidence for antiinflammatory actions. Pharmacotherapy 2005;25:1213–29.
Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, et al. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 2009;296:R208–16.
Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol 2015;179:137–45.
Abdel-Fattah MM, Salama AAA, Shehata BA, Ismaiel IE. The potential effect of the angiotensin II receptor blocker telmisartan in regulating OVA-induced airway remodeling in experimental rats. Pharmacol Rep 2015;67:943–51.
Dandona P, Kumar V, Aljada A, Ghanim H, Syed T, Hofmayer D, et al. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-κB, in mononuclear cells of normal subjects: evidence of an antiinflammatory action. J Clin Endocrinol Metab 2003;88:4496–501.
Suzuki Y, Gómez-Guerrero C, Shirato I, López-Franco O, Hernández-Vargas P, Sanjuán G, et al. Susceptibility to T cell-mediated injury in immune complex disease is linked to local activation of renin-angiotensin system: the role of NF-AT pathway. J Immunol 2002;169:4136–46.
Silva-Filho JL, Souza MC, das Graças Henriques M, Morrot A, Savino W, Nunes MP, et al. AT1 receptor-mediated angiotensin II activation and chemotaxis of T lymphocytes. Mol Immunol 2011;48:1835–43.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med 2007;204:2449–60.
Lapteva N, Ide K, Nieda M, Ando Y, Hatta-Ohashi Y, Minami M, et al. Activation and suppression of renin-angiotensin system in human dendritic cells. Biochem Biophys Res Commun 2002;296:194–200.
Nahmod K, Gentilini C, Vermeulen M, Uharek L, Wang Y, Zhang J, et al. Impaired function of dendritic cells deficient in angiotensin II type 1 receptors. J Pharmacol Exp Ther 2010;334:854–62.
Berin MC. Future therapies for IgE-mediated food allergy. Curr Pediatr Rep 2014;2:119–26.
Burks AW, Sampson HA, Plaut M, Lack G, Akdis CA. Treatment for food allergy. J Allergy Clin Immunol 2018;141:1–9.
Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol 1998;8:168–75.
Viñas P, Martínez-Castillo N, Campillo N, Hernández-Córdoba M. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J Chromatogr A 2011;1218:639–46.
Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res 2007;1173:117–25.
Markovic ZS, Mentus SV, Dimitric Markovic JM. Electrochemical and density functional theory study on the reactivity of fisetin and its radicals: implications on in vitro antioxidant activity. J Phys Chem A 2009;113:14170–9.
Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res 2008;31:1303–11.
Kim SC, Kang SH, Jeong SJ, Kim SH, Ko HS. Inhibition of c-Jun N-terminal kinase and nuclear factor kappa B pathways mediates fisetin-exerted anti-inflammatory activity in lipopolysccharide-treated RAW264.7 cells. Immunopharmacol Immunotoxicol 2012;34:645–50.
Wu M-Y, Hung S-K, Fu S-L. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J Agric Food Chem 2011;59:10496–504.
Goh FY, Upton N, Guan S, Cheng C, Shanmugam MK, Sethi G, et al. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur J Pharmacol 2012;679:109–16.
Patel P. Telmisartan: clinical evidence across the cardiovascular and renal disease continuum. Drugs Ther Perspect 2017;33:77–87.
Fujita H, Sakamoto T, Komatsu K, Fujishima H, Morii T, Narita T, et al. Reduction of circulating superoxide dismutase activity in type 2 diabetic patients with microalbuminuria and its modulation by telmisartan therapy. Hypertens Res 2011;34:1302.
Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Yamada S, Ueda Y, et al. Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. Am J Med Sci 2012;343:46–51.
Link A, Lenz M, Legner D, Böhm M, Nickenig G. Telmisartan inhibits β2-integrin MAC-1 expression in human T-lymphocytes. J Hypertens 2006;24:1891–8.
Noone D, Licht C. Chronic kidney disease: a new look at pathogenetic mechanisms and treatment options. Pediatr Nephrol 2014;29:779–92.
Hammad H, de Heer HJ, Soullie T, Angeli V, Trottein F, Hoogsteden HC, et al. Activation of peroxisome proliferator-activated receptor-gamma in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol 2004;164:263–71.
Matsui T, Yamashita H, Mori M, Tanaka H, Inagaki N. Eppikajutsuto protects against food allergy induced by Ovalbumin in a murine model. Int Arch Allergy Immunol 2017;173:71–83.
Bie J, Hessel E, Ark I, Esch B, Hofman G, Nijkamp F, et al. Effect of dexamethasone and endogenous corticosterone on airway hyperresponsiveness and eosinophilia in the mouse. Br J Pharmacol 1996;119:1484–90.
Haraguchi T, Iwasaki K, Takasaki K, Uchida K, Naito T, Nogami A, et al. Telmisartan, a partial agonist of peroxisome proliferator-activated receptor g, improves impairment of spatial memory and hippocampal apoptosis in rats treated with repeated cerebral ischemia. Brain Res 2010;1353:125–32.
Jo JH, Jo JJ, Lee J-M, Lee S. Identification of absolute conversion to geraldol from fisetin and pharmacokinetics in mouse. J Chromatogr B 2016;1038:95–100.
Yamashita H, Takahashi K, Tanaka H, Nagai H, Inagaki N. Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy 2012;67:201–9.
Duncker SC, Philippe D, Martin-Paschoud C, Moser M, Mercenier A, Nutten S. Nigella sativa (black cumin) seed extract alleviates symptoms of allergic diarrhea in mice, involving opioid receptors. PloS One 2012;7:e39841.
Henry J, Kurec A. The clinical laboratory: organization, purposes, and practice. Clinical diagnosis and management by laboratory methods. 20th ed Philadelphia: WB Saunders; 2001. p. 13–7.
Wang CC, Lin YR, Liao MH, Jan TR. Oral supplementation with areca-derived polyphenols attenuates food allergic responses in ovalbumin-sensitized mice. BMC Complement Altern Med 2013;13:154.
Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques, expert consult: online and print, 7: bancroft’s theory and practice of histological techniques. Elsevier Health Sciences; 2013.
d’Ettorre G, Baroncelli S, Micci L, Ceccarelli G, Andreotti M, Sharma P, et al. Reconstitution of intestinal CD4 and Th17 T cells in antiretroviral therapy suppressed HIV-infected subjects: implication for residual immune activation from the results of a clinical trial. PLoS One 2014;9:e109791.
Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996;383:787.
Dourado LPA, da Silva Saldanha JC, Gargiulo DL, MdLM Noviello, Brant CC, Reis MLC, et al. Role of IL-4 in aversion induced by food allergy in mice. Cell Immunol 2010;262:62–8.
Singh A, Holvoet S, Mercenier A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin Exp Allergy 2011;41:1346–59.
Higa S, Hirano T, Kotani M, Matsumoto M, Fujita A, Suemura M, et al. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J Allergy Clin Immunol 2003;111:1299–306.
Park H-H, Lee S, Oh J-M, Lee M-S, Yoon K-H, Park BH, et al. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol Res 2007;55:31–7.
Nagai K, Takahashi Y, Mikami I, Fukusima T, Oike H, Kobori M. The hydroxyflavone, fisetin, suppresses mast cell activation induced by interaction with activated T cell membranes. Br J Pharmacol 2009;158:907–19.
Algaem MA, Numan IT, Hussain SA. Effects of Valsartan and Telmisartan on the LungTissue histology in sensitized rats. Am J Pharmacol Sci 2013;1:56–60.
Veerappan A, Reid AC, Estephan R, O’Connor N, Thadani-Mulero M, Salazar-Rodriguez M, et al. Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci 2008;105:1315–20.
Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 2008;29:367–74.
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2010;2:247–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elkholy, R., Balaha, M., El-Anwar, N. et al. Fisetin and telmisartan each alone or in low-dose combination alleviate OVA-induced food allergy in mice. Pharmacol. Rep 71, 330–337 (2019). https://doi.org/10.1016/j.pharep.2018.12.009
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2018.12.009