Abstract
Study Design
Prospective longitudinal study of growth modulation system for early adolescent idiopathic scoliosis (AIS), consecutive case series from first human use to skeletal maturity, fusion, or five years postoperation.
Objectives
Determine adverse events and curvature changes to end of study; examine factors most likely to explain variability in curve changes.
Summary of Background
Pilot clinical safety study was performed under US Food and Drug Administration (FDA) Investigational Device Exemption (IDE). Safety and radiographic results were previously reported to 24 months postoperation.
Methods
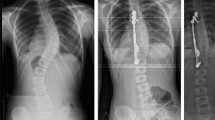
Subjects with early AIS underwent thoracoscopic placement of titanium clip-screw devices designed to modify growth asymmetrically. Eligibility was based on high risk of progression to 50°: single major thoracic curve 25°–40°, Risser 0, open triradiate cartilages, and premenarchal if female. Six subjects, the maximum allowed, enrolled. Adverse events (AEs), clinical outcomes, and curvatures were systematically collected. Disc heights, vertebral heights, and implant-bone contact areas were assessed.
Results
Consecutive subjects enrolled, aged 12.1 years (±1.7), three were female. AEs from two to five years postoperation included deformity changes leading to a second surgery in three patients: two for posterior spinal fusion, and one for thoracoscopic removal of half the implants for overcorrection. In the latter case, overcorrection appeared halted for duration of study. One patient, whose curve exceeded 50° at age 18 years, did not choose fusion. Major thoracic curves were 34° (±3°) preoperatively and 42° (±20°) at end of study.
Conclusions
In a study of spine growth modulation in patients with early AIS with high risk of progression, at skeletal maturity or five years postoperation, major thoracic curves of half progressed to >50°, whereas curves of the other half remained <40°, below fusion indications. Removal of selected implants may halt overcorrection. The next, pivotal, study phase was approved by FDA.
Level of Evidence
Level IV, prospective case series under stringent regulatory controls.
Similar content being viewed by others
References
Ponseti IV, Friedman B. Prognosis in idiopathic scoliosis. J Bone Joint Surg Am 1950;32:381–95.
Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am 1984;66:1061–71.
Charles YP, Daures JP, de Rosa V, et al. Progression risk of idiopathic juvenile scoliosis during pubertal growth. Spine 2006;31:1933–42.
Sanders JO, Browne RH, McConnell SJ, et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am 2007;89:64–73.
Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am 2008;90:540–53.
Sitoula P, Verma K, Holmes Jr L, et al. Prediction of curve progression in idiopathic scoliosis: validation of the Sanders skeletal maturity staging system. Spine 2015;40:1006–13.
Karol LA, Johnston CE, Browne RH, et al. Progression of the curve in boys who have idiopathic scoliosis. J Bone Joint Surg Am 1993;75:1804–10.
Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am 1995;77:815–22.
Emans JB, Kaelin A, Bancel P, et al. The Boston bracing system for idiopathic scoliosis. Follow-up results in 295 patients. Spine 1986;11:792–801.
Little DG, Song KM, Katz D, et al. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am 2000;82:685–93.
Song KM, Little DG. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop 2000;20:286–8.
Ryan PM, Puttler EG, Stotler WM, et al. Role of the triradiate cartilage in predicting curve progression in adolescent idiopathic scoliosis. J Pediatr Orthop 2007;27:671–6.
Karol LA. Effectiveness of bracing in male patients with idiopathic scoliosis. Spine 2001;26:2001–5.
Richards BS, Bernstein RM, D’Amato CR, et al. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine 2005;30:2068–75.
Katz DE, Herring JA, Browne RH, et al. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am 2010;92:1343–52.
Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 2013;369:1512–21.
Karol LA, Virostek D, Felton K, et al. The effect of the Risser stage on bracing outcome in adolescent idiopathic scoliosis. J Bone Joint Surg Am 2016;98:1253–9.
Arkin AM, Katz JF. The effects of pressure on epiphyseal growth; the mechanism of plasticity of growing bone. J Bone Joint Surg Am 1956;38A:1056–76.
Roaf R. Vertebral growth and its mechanical control. J Bone Joint Surg Br 1960;42:40–59.
Harrington PR. Is scoliosis reversible? In vivo observations of reversible morphological changes in the production of scoliosis in mice. Clin Orthop Relat Res 1976;116:103–11.
Stokes IAF, Aronsson DD, Urban JPG. Biomechanical factors influencing progression of angular skeletal deformities during growth. Eur J Exp Musculoskel Res 1994;3:51–60.
Stokes IA, Burwell RG, Dangerfield PH. IBSE: biomechanical spinal growth modulation and progressive adolescent scoliosis—a test of the “vicious cycle” pathogenic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis 2006; 1:16.
Bylski-Austrow DI, Glos DL, Wall EJ, Crawford AH. Scoliosis vertebral growth plate histomorphometry: comparisons to controls, growth rates, and compressive stresses. J Orthop Res 2018;36:2450–9.
Mente PL, Aronsson DD, Stokes IAF, Iatridis JC. Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res 1999;17:518–24.
Stokes IA, Mente PL, Iatridis JC, et al. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am 2002;84:1842–8.
Braun JT, Ogilvie JW, Akyuz E, et al. Fusionless scoliosis correction using a shape memory alloy staple in the anterior thoracic spine of the immature goat. Spine 2004;29:1980–9.
Wall EJ, Bylski-Austrow DI, Kolata RJ, Crawford AH. Endoscopic mechanical spinal hemiepiphysiodesis modifies spine growth. Spine 2005;30(10):1148–53.
Stokes IA, Aronsson DD, Dimock AN, et al. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res 2006;24:1327–34.
Newton PO, Upasani VV, Farnsworth CL, et al. Spinal growth modulation with use of a tether in an immature porcine model. J Bone Joint Surg Am 2008;90:2695–706.
Bylski-Austrow DI, Wall EJ, Glos DL, et al. Spinal hemiepiphysiodesis decreases the size of vertebral growth plate hypertrophic zone and cells. J Bone Joint Surg Am 2009;91:584–93.
Clinicaltrials.gov [Internet]. Identifier: NCT01465295: evaluate initial safety of the HemiBridge System in guided spinal growth treatment of progressive idiopathic scoliosis. Available at: http://clinicltrials.gov/ct2/show/NCT01465295. Accessed February 5, 2018.
Wall EJ, Reynolds JE, Jain VV, et al. Spine growth modulation in early adolescent idiopathic scoliosis: two year results of prospective US FDA IDE pilot clinical safety study of titanium clip-screw implant. Spine Deform 2017;5:314–24.
Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford: Stanford University Press; 1959. p. 266–70.
Jain V, Lykissas M, Trobisch P, et al. Surgical aspects of spinal growth modulation in scoliosis correction. Instr Course Lect 2014;63:335–44.
Wall EJ, Bylski-Austrow DI, Reynolds JE, et al. Growth modulation techniques: titanium clip-screw implant system (HemiBridge). In: The growing spine. Berlin: Springer; 2016. p. 769–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author disclosures: EJW (grants from FDA and Ohio Department of Development; personal fees, nonfinancial support, and other from SpineForm LLC, during the conduct of the study; in addition, EJW has patents 8021403, 6746450, 7481830, and 6,746,450 licensed to SpineForm LLC), VVJ (other from SpineForm LLC; personal fees from Medtronic Inc., during the conduct of the study), AHC (nonfinancial support from SpineForm LLC, during the conduct of the study; in addition, AHC has a patent 9,072,554 licensed to SpineForm LLC), DIBA (grants and nonfinancial support from SpineForm LLC; grants from FDA, via SpineForm LLC, and Ohio Department of Development, during the conduct of the study; in addition, DIBA has patents 6,746,450, 7,481,830, and 8,021,403 licensed to SpineForm LLC), JER (grants and personal fees from SpineForm LLC; grants from FDA, via SpineForm LLC, and Ohio Department of Development, during the conduct of the study; personal fees from Reynolds Consulting Inc. and ApiFix Inc., outside the submitted work; in addition, JER has a patent 8062294 issued, and patents 9072554 and 8,021,403 licensed to SpineForm LLC).
Device Status Statement: The devices that are the subject of this manuscript were evaluated as part of a US FDA Investigational Device Exemption (IDE) for the intended use of guided spinal growth treatment of progressive idiopathic scoliosis (IS). The test article is intended for anterior-lateral Cobb to Cobb fixation across the growth plates from T3 to L1 with placement through thoracoscopic surgery.
Regulatory status: IRB approved, FDA Investigational Device Exemption (IDE) and Humanitarian Use Device (HUD); http://clinicaltrials.gov/show/NCT01465295. US Food and Drug Administration, Evaluate Initial Safety of the HemiBridge System in Guided Spinal Growth Treatment of Progressive Idiopathic Scoliosis, 2013 [First posted November 4, 2011; Current update posted February 5, 2018], device approved for use in European Union (EU CE Marking) for the labeled indications.
Rights and permissions
About this article
Cite this article
Wall, E.J., Jain, V.V., Crawford, A.H. et al. Spine Growth Modulation in Early Adolescent Idiopathic Scoliosis: Prospective US FDA IDE Pilot Study of Titanium Clip-Screw Implant at Two to Five Years. Spine Deform 7, 899–909 (2019). https://doi.org/10.1016/j.jspd.2019.02.008
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jspd.2019.02.008