Abstract
Abstract
After tethered growth or sham surgery, spinal motion segments underwent microtomography to determine physeal and disc 3-dimensional (3D) morphology. Instrumented and contralateral sides of tether and sham surgical groups were compared.
Objectives
To determine the 3D morphological effects of growth modulation via anterolateral tethering on vertebral physeal and intervertebral disc morphology in a rapidly growing bovine model.
Summary of background data
Growth modulation acts through physeal loading. Providing a promising alternative to arthrodesis for scoliosis correction, tethering vertebral growth maintains further growth (open/functioning physes) and motion (disc integrity). Standard physeal and disc evaluation using histology reduces 3D geometries to single planar samples.
Methods
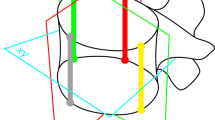
Five-week-old calves received anterolateral flexible spinal tethers (n = 6) or sham surgeries (n = 6) followed by 6 months of growth. Individual motion segments were imaged by microtomograph (36 μm). Physeal space and disc space thickness maps were generated from surface reconstructions. Normalized thickness differences were compared between instrumented and contralateral sides of tether and sham groups (analysis of variance, p <.05). Physeal closure was estimated and regions of bony bridging were marked closed. Results: Tethering caused significant physeal thickness reduction on the instrumented side compared with the contralateral side (7.6% ± 2.0%; p =.0002). This reduction was greater (p =.003) in tethered physes than in the sham, which demonstrated no reduction (0.8% ± 3.7%; p =.6). Small regions of physeal closure were observed in sham and tether groups (medians of 1.4% and 0.1% and maximums of 6.8% and 2.7%, respectively). Tethered discs were 29% thinner than sham, but demonstrated no contralateral to instrumented-side thickness difference (5.2% difference; p =.3).
Conclusions
Tethering resulted in thinner physes on the tethered side without notable physeal closure. With no side differences in the sham group, tethering apparently applied instrument-sided compressive forces. Tethering also resulted in thinner discs, although they were apparently. Producing consistent histological samples is difficult; misaligned slices may lead to inaccurate conclusions. Evaluating entire physes or discs produces more robust results.
Similar content being viewed by others
References
Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 2005;30:1082–5.
Little DG, Song KM, Katz D, et al. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am 2000;82:685–93.
Charles YP, Daures JP, de Rosa V, et al. Progression risk of idiopathic juvenile scoliosis during pubertal growth. Spine 2006;31:1933–42.
Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am 1983;65:447–55.
Betz RR, Kim J, D’Andrea LP, et al. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: a feasibility, safety, and utility study. Spine 2003;28:S255–65.
Betz RR, Ranade A, Samdani AF, et al. Vertebral body stapling: a fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine 2010;35:169–76.
Lavelle WF, Samdani AF, Cahill PJ, et al. Clinical outcomes of nitinol staples for preventing curve progression in idiopathic scoliosis. J Pediatr Orthop 2011;31(Suppl 1):S107–13.
Aronsson DD, Stokes IA. Nonfusion treatment of adolescent idiopathic scoliosis by growth modulation and remodeling. J Pediatr Orthop 2011;31(Suppl 1):S99–106.
Crawford CH, Lenke LG. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: a case report. J Bone Joint Surg Am 2010;92:202–9.
Sanders JO, D’Astous J, Fitzgerald M, et al. Derotational casting for progressive infantile scoliosis. J Pediatr Orthop 2009;29:581–7.
Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis: a prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am 1995;77:815–22.
Maruyama T. Bracing adolescent idiopathic scoliosis: a systematic review of the literature of effective conservative treatment looking for end results5 years after weaning. Disabil Rehabil 2008;30:786–91.
Maruyama T, Grivas TB, Kaspiris A. Effectiveness and out comes of brace treatment: asystematic review. Physiother Theory Pract 2011;27:26–42.
Wall EJ, Bylski-Austrow DI, Kolata RJ, et al. Endoscopic mechanical spinal hemiepiphysiodesis modifies spine growth. Spine 2005;30:1148–53.
Schwab F, Patel A, Lafage V, et al. A porcine model for progressive thoracic scoliosis. Spine 2009;34:E397–404.
Braun JT, Ogilvie JW, Akyuz E, et al. Experimental scoliosis in an immature goat model: a method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine 2003;28:2198–203.
Braun JT, Akyuz E, Ogilvie JW. The use of animal models in fusionless scoliosis investigations. Spine 2005;30(Suppl 17):S35–45.
Braun JT, Hines JL, Akyuz E, et al. Relative versus absolute modulation of growth in the fusionless treatment of experimental scoliosis. Spine 2006;31:1776–82.
Braun JT, Hoffman M, Akyuz E, et al. Mechanical modulation of vertebral growth in the fusionless treatment of progressive scoliosis in an experimental model. Spine 2006;31:1314–20.
Newton PO, Fricka KB, Lee SS, et al. Asymmetrical flexible tethering of spine growth in an immature bovine model. Spine 2002;27:689–93.
Newton PO, Farnsworth CL, Faro FD, et al. Spinal growth modulation with an anterolateral flexible tether in an immature bovine model: disc health and motion preservation. Spine 2008;33:724–33.
Newton PO, Upasani VV, Farnsworth CL, et al. Spinal growth modulation with use of a tether in an immature porcine model. J Bone Joint Surg Am 2008;90:2695–706.
Upasani VV, Farnsworth CL, Chambers RC, et al. Intervertebral disc health preservation after six months of spinal growth modulation. J Bone Joint Surg Am 2011;93:1408–16.
Stokes IA. Analysis and simulation of progressive adolescent scoliosis by biomechanical growth modulation. Eur Spine J 2007;16:1621–8.
Bylski-Austrow DI, Wall EJ, Glos DL, et al. Spinal hemiepiphysiodesis decreases the size of vertebral growth plate hypertrophic zone and cells. J Bone Joint Surg Am 2009;91:584–93.
Aronsson DD, Stokes IA, McBride C. The role of remodeling and asymmetric growth in vertebral wedging. Stud Health Technol Inform 2010;158:11–5.
Stokes IA, Mente PL, Iatridis JC, et al. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am 2002;84:1842–8.
Stokes IA, Clark KC, Farnum CE, et al. Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone 2007;41:197–205.
Chay E, Patel A, Ungar B, et al. Impact of unilateral corrective tethering on the histology of the growth plate in an established porcine model for thoracic scoliosis. Spine 2012;37:E883–9.
Stokes IA, Windisch L. Vertebral height growth predominates over intervertebral disc height growth in adolescents with scoliosis. Spine 2006;31:1600–4.
Dahia CL, Mahoney EJ, Durrani AA, et al. Postnatal growth, differentiation, and aging of the mouse intervertebral disc. Spine 2009;34:447–55.
Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pul-posus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS One 2012;7:–35944.
Hunt KJ, Braun JT, Christensen BA. The effect of two clinically relevant fusionless scoliosis implant strategies on the health of the intervertebral disc: analysis in an immature goat model. Spine 2010;35:371–7.
Stokes IA, McBride C, Aronsson DD, et al. Intervertebral disc changes with angulation, compression and reduced mobility simulating altered mechanical environment in scoliosis. Eur Spine J 2011;20:1735–44.
Sevinc O, Barut C, Is M, et al. Influence of age and sex on lumbar vertebral morphometry determined using sagittal magnetic resonance imaging. Ann Anat 2008;190:277–83.
Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine 2004;29:2724–32.
Stokes IA, Mente PL, Iatridis JC, et al. Growth plate chondrocyte enlargement modulated by mechanical loading. Stud Health Technol Inform 2002;88:378–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author disclosures: PON (grant from the Scoliosis Research Society; board membership with Pediatric Orthopaedics Society of North America, Setting Scoliosis Straight Foundation; grants from DePuy, EOS Imaging, Pediatric Orthopaedic Society of North America; payment for lectures including service on speakers bureaus from DePuy; royalties from Thieme Publishing; stock/stock options from NuVasive; travel/accommodations/ meeting expenses from DePuy); DAG (grants from EOS Imaging, SRS, POS-NA, Navy Medical Center, K2M, DePuy Spine, KFx, Absorption Systems, MediGald; stock/stock options from Mako, Mankind, Alphatec, NuVasive); JDD (none); CLF (none).
This study was funded by a grant from the Scoliosis Research Society and from support from the Children’s Specialists of San Diego.
The animal work presented in this report was performed under the supervision of the University of California San Diego Institutional Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Newton, P.O., Glaser, D.A., Doan, J.D. et al. 3D Visualization of Vertebral Growth Plates and Disc: The Effects of Growth Modulation. Spine Deform 1, 313–320 (2013). https://doi.org/10.1016/j.jspd.2013.07.005
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jspd.2013.07.005