Abstract
Objective
Intrauterine growth retardation (IUGR) is an important cause of prenatal and neonatal morbidity, and neurologic abnormalities. Although several animal models of IUGR have been developed for scientific investigation, few models approximate the pathophysiology in human fetal growth failure resulting from pregnancy-induced hypertension and preeclampsia. We developed an animal model of IUGR in which fetal growth restriction was induced by administering a synthetic thromboxane A2 analogue (STA2) to the mother.
Methods
Timed pregnant Sprague-Dawley rats were used in this study. STA2 was delivered into the peritoneal cavity of the pregnant female at a rate of 20 ng/h from day 13 of pregnancy. The effectiveness of this model was evaluated by monitoring the overall growth of the fetuses and neonates and measuring the weight and biochemical composition of individual organs.
Results
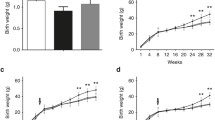
Fetuses and neonates from the STA2 group showed a highly significant weight reduction throughout the observation period from day 19 of gestation to postnatal day 7. Weight reduction near and at term exceeded 10% and became more pronounced during the first week after birth. Fetuses on the 20th gestational day exhibited a pattern of growth retardation characteristic of asymmetrical IUGR in which the weight reduction was prominent in the liver with relative sparing of the brain. However, the decrease in brain weight was more than 10%. The protein, DNA, and RNA contents of the liver were lower in the STA2 group. The protein content of the forebrain and brainstem also decreased significantly in the STA2 group compared with the control; however, the DNA content of the forebrain was higher in the STA2 group.
Conclusions
This animal model may mimic human IUGR more closely than previous models because the growth restriction is induced in a truly chronic manner.
Similar content being viewed by others
References
Aucott SW, Donohue PK, Northington FJ. Increased morbidity in severe early intrauterine growth restriction. J Perinatol 2004;24;435–440.
Bergvall N, Iliadou A, Johansson S, Tuvemo T, Cnattingius S. Risks for low intellectual performance related to being born small for gestational age are modified by gestational age. Pediatrics 2006;117:e460–e467.
Martorell R, Ramakrishnan U, Schroeder DG, Melgar P, Neufeld L. Intrauterine growth retardation, body size, body composition and physical performance in adolescence. Eur J Clin Nutr 1998;52(Suppl 1):S43–S52.
Strauss RS, Dietz WH. Effects of intrauterine growth retardation in premature infants on early childhood growth. J Pediatr 1997;130:95–102.
Schreuder MF, Fodor M, van Wijk JA, Delemarre-van de Waal HA. Association of birth weight with cardiovascular parameters in adult rats during baseline and stressed conditions. Pediatr Res 2006;59:126–130.
Simmons R. Developmental origins of adult metabolic disease: Concepts and controversies. Trends Endocrinol Metab 2005;16:390–394.
Brodszki J, Lanne T, Marsal K, Ley D. Impaired vascular growth in late adolescence after intrauterine growth restriction. Circulation 2005;111;2623–2628.
Wigglesworth JS. Experimental growth retardation in the foetal rat. J Pathol Bacteriol 1964;88:1–13.
Roux JM, Tordet-Caridroit C, Chanez C. Studies on experimental hypotrophy in the rat. I. Chemical composition of the total body and some organs in the rat foetus. Biol Neonate 1970;15:342–347.
Cha CJ, Gelar di NL, Oh W. Growth and cellular composition in rats with intrauterine growth retardation: Effects of postnatal nutrition. J Nutr 1987;117:1463–1468.
Hayashi TT, Dorko ME. A rat model for the study of intrauterine growth retardation. Am J Obstet Gynecol 1988;158:1203–1207.
Lueder FL, Ogata ES. Uterine artery ligation in the maternal rat alters fetal tissue glucose utilization. Pediatr Res 1990;28:464–468.
Van Geijn HP, Kaylor WM Jr, Nico la KR, Zuspan FP. Induction of severe intrauterine growth retardation in the Sprague-Dawley rat. Am J Obstet Gynecol 1980;137:43–47.
Rosso P. Maternal-fetal exchange during protein malnutrition in the rat. Placental transfer of alpha-amino isobutyric acid. J Nutr 1977;107:2002–2005.
McKenzie JM, Fosmire GJ, Sandstead HH. Zinc deficiency during the latter third of pregnancy: Effects on fetal rat brain, liver, and placenta. J Nutr 1975;105:1466–1475.
Streissguth AP, Landesman-Dwyer S, Martin JC, Smith DW. Teratogenic effects of alcohol in humans and laboratory animals. Science 1980;209:353–361.
Chaube S, Swinyard CA. Cellular and biochemical aspects of growth retardation in rat fetuses induced by maternal administration of selected anticancer agents. Teratology 1975;12:259–270.
Wallenburg HC, Rotmans N. Prevention of recurrent idiopathic fetal growth retardation by low-dose aspirin and dipyridamole. Am J Obstet Gynecol 1987;157:1230–1235.
Magness RR, Mitchell MD, Rosenfeld CR. Uteroplacental production of eicosanoids in ovine pregnancy. Prostaglandins 1990;39:75–88.
Sibai BM. An aspirin a day to prevent prematurity. Clin Perinatol 1992;19:305–317.
Mills JL, DerSimonian R, Raymond E, et al. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: A multicenter prospective study. JAMA 1999;282:356–362.
Rocca B, Loeb AL, Strauss JF 3rd, et al. Directed vascular expression of the thromboxane A2 receptor results in intrauterine growth retardation. Nat Med 2000;6:219–221.
Pradelles P, Grassi J, Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: An alternative to radioimmunoassay. Anal Chem 1985;57:1170–1173.
Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 1965;17:381–387.
Schmidt G, Thannhauser SJ. A method for determination of deoxyribonucleic acid, ribonucleic acid, and phosphoproteins in animal tissues. J Biol Chem 1945;161:83–89.
Zamenhof S, Bursztyn H, Rich K, Zamenhof PJ. The determination of deoxyribonucleic acid and of cell number in brain. J Neurochem 1964;11:505–509.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275.
Ergaz Z, Avgil M, Ornoy A. Intrauterine growth restriction-etiology and consequences: What do we know about the human situation and experimental animal models? Reprod Toxicol 2005;20:301–322.
Ishimitsu T, Uehara Y, Ishii M, Sugimoto T. Enhanced generation of vascular thromboxane A2 in spontaneously hypertensive rats and its role in the rapid proliferation of vascular smooth muscle cells. Am J Hypertens 1988;1:38S–40S.
Yusuf K, Smith SD, Levy R, et al. Thromboxane A(2) limits differentiation and enhances apoptosis of cultured human trophoblasts. Pediatr Res 2001;50:203–209.
Younoszai MK, Peloso J, Haworth JC. Fetal growth retardation in rats exposed to cigarette smoke during pregnancy. Am J Obstet Gynecol 1969;104:1207–1213.
Nitzan M, Orloff S, Chrzanowska BL, Schulman JD. Intrauterine growth retardation in renal insufficiency: An experimental model in the rat. Am J Obstet Gynecol 1979;133:40–43.
de Grauw TJ, Myers R E, Scott WJ. Fetal growth retardation in rats from different levels of hypoxia. Biol Neonate 1986;49:85–89.
Jacobson M. Histogenesis and morphogenesis of cortical stuctures. In: Jacobson M, ed. Developmental Neurobiology. 3rd ed. New York: Plenum, 1991:401—52.
Hill DE, Myers RE, Holt AB, Scott RE, Cheek DB. Fetal growth retardation produced by experimental placental insufficiency in the rhesus monkey. II. Chemical composition of the brain, liver, muscle and carcass. Biol Neonate 1971;19:68–82.
O W, Guy JA. Cellular growth in experimental intrauterine growth retardation in rats. J Nutr 1971;101:1631–1633.
Bernal A, Morales M, Feria-Velasco A, Chew S, Rosado A. Effect of intrauterine growth retardation on the biochemical maturation of brain synaptosomes in the rat. J Nutr 1974;104:1157–1164.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a Grant-in-Aid (No. 17591139) for Scientific Research from the Japan Society for the Promotion of Science.
Rights and permissions
About this article
Cite this article
Hayakawa, M., Takemoto, K., Nakayama, A. et al. An Animal Model of Intrauterine Growth Retardation Induced by Synthetic Thromboxane A2. Reprod. Sci. 13, 566–572 (2006). https://doi.org/10.1016/j.jsgi.2006.09.007
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2006.09.007