Abstract
Objective
To determine during mid-trimester amniocentesis if elevated concentrations of ADAM-8 (A Disintegrin And Metalloprotease 8) and/or cortisol can recognize women at risk for spontaneous preterm delivery.
Methods
The study involved 312 women who underwent mid-trimester amniocentesis. Thirteen patients, who progressed to preterm delivery, were matched with 21 controls for age, parity, gestational age at amniocentesis, and year of amniocentesis. ADAM-8 and cortisol levels were measured by enzyme-linked immunosorbent assay and radioimmunoassay, respectively.
Results
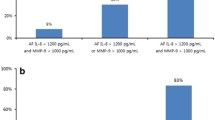
ADAM-8 mean amniotic fluid concentrations were significantly higher in women with preterm delivery than in women delivering at term (mean 1213.9 [SE 96.7] pg/mL [range, 780 to 1854 pg/mLJ vs mean 937.2 [SE 50.3] pg/mL [range, 486 to 1508 pg/mL], P <.02). Amniotic fluid ADAM-8 concentrations higher than 1149 pg/mL had the highest specificity and odds ratio (OR) in the identification of the women with increased risk for preterm delivery (sensitivity 61.5%; specificity 81.7%; OR, 9.6 [95% confidence interval (Cl), 1.8 to 50.3]). Women with preterm delivery had suggestively higher amnioticfiuid concentrations of cortisol (mean 1.3 [SE 0.2] μg/dL [range, 0.4 to 2.2 μg/dL]) than women delivering at term (mean 1.0 [SE 0.09] μLg/dL [range, 0.6 to 1.7 μg/dL], P <.07). Furthermore, cortisol levels were positively correlated with ADAM-8 levels (Spearman’s r =.418, P <.014).
Conclusions
Elevated mid-trimester amnioticfiuid ADAM-8 concentrations possibly are a riskfactor for preterm delivery, particularly if ADAM-8 levels are greater than 1149 pg/mL. Potential intrauterine inflammation is also associated with suggestively increased amniotic fluid cortisol levels.
Similar content being viewed by others
References
Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol 1979;54:226–20.
Vintzileos AM, Campbell WA, Nochimson DJ, Connolly ME, Fuenfer MM, Hoehn GJ. The fetal biophysical profile in patients with premature rupture of the membranes—An early predictor of fetal infection. Am J Obstet Gynecol 1985;152:510–6.
Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229–37.
Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, while blood cell count, interleukin-6, and Gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51.
Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol 1998;179:172–8.
Yoon BH, Oh SY, Romero R, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001;185:1162–7.
Schettler A, Thorn H, Jockusch BM, Tschesche H. Release of proteinases from stimulated polymorphonuclear leukocytes. Evidence for subclasses of the main granule types and their association with cytoskeletal components. Eur J Biochem 1991;197:197–202.
Romero R, Munoz H, Gomez R, et al. Two thirds of spontaneous abortion/fetal deaths after amniocentesis are the results of a pre-existing subclinical inflammatory process of the amniotic cavity [abstr 24]. Am J Obstet Gynecol 1995;172:261.
Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol 1996;175:830–3.
Spong CY, Ghidini A, Sherer DM, Pezzullo JC, Ossandon M, Eglinton GS. Angiogenin: A marker for preterm delivery in midtrimester amniotic fluid. Am J Obstet Gynecol 1997;176:415–8.
Wenstrom KD, Andrews WW, Hauth JC. Goldenberg RL, DuBard MB, Cliver SP. Elevated mid-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–50.
Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S. CD156 (human ADAM-8): Expression, primary amino acid sequence, and gene location. Genomics 1997;41:56–62.
Yoshida S, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol 1990;2:585–91.
Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain: Multipotential functions in cell-cell and cell-matrix interactions. J Cell Biol 1995;131:275–8.
Choi SJ, Han JH, Roodman GD. ADAM-8: A novel osteoclast stimulating factor. J Bone Miner Res 2001;16:814–22.
Primakoff P, Myles DG. The ADAM gene family: Surface proteins with adhesion and protease activity. Trends Genet 2000;16:83–7.
Kataoka M, Yoshiyama K, Matsuura K, Hijiya N, Higuchi Y, Yamamoto S. Structure of the murine CD156 gene, characterization of its promoter, and chromosomal location. J Biol Chem 1997;29:18209–15.
Aoki N, Ohno Y, Omamura M. Physiological interactions between the immune and endocrine system: Are cytokines hormones? Med Sci Res 1990;18:195–201.
Riechlin S. Neuroendocrine-immune interactions. N Engl J Med 1993;329:1246–53.
Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: Facts and hypotheses. Endocr Rev 1996;17:64–102.
Chaim W, Mazor M. The relationship between hormones and human parturition. Arch Gynecol Obstet 1998;262:43–51.
Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: Evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol 2000;182:1404–12.
Gardosi J, Chang A, Kaylan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet 1992;339:283–7.
Dudley DJ, Trautman MS. Infection, inflammation, and contractions: The role of cytokines in the pathophysiology of preterm labor. Semin Reprod Endocrinol 1994;12:263–72.
McGregor JA, Lawellin D, Franco-Buff A, Todd JK, Makowski EC. Protease production by microorganisms associated with reproductive tract infection. Am J Obstet Gynecol 1986;154:109–14.
McGregor JA, French JI, Lawellin D, Franco-Buff A, Smith C, Todd JK. In vitro study of bacterial protease-induced reduction of chorioamniotic membrane strength and elasticity. Obstet Gynecol 1986;69:167–74.
Jiang W, Bond JS. Families of metalloendopeptidases and their relationships. FEBS Lett 1992;312:110–4.
Amour A, Knight CG, English WR, et al. The enzymatic activity of ADAM-8 and ADAM9 is not regulated by TIMPs. FEBS Lett 2002;31:154–8.
Yamamoto S, Higuchi Y, Yoshiyama K, et al. ADAM family proteins in the immune system. Immunol Today 1999;20:278–84.
Sweep F, Rijnkels C, Hermus A. Activation of the hypothalamus-pituitary-adrenal axis by cytokines. Acta Endocrinol (Copenh) 1991;125 Suppl 1:84–91.
McDonald TJ, Nathanielsz PW. Bilateral destruction of fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 1991;165:764–70.
Beverley EP, John P, Ronald LD, et al. Cortisol in amniotic fluid during human gestation. J Clin Endocrinol Metab 1975;40:164–8.
Mazor M, Hershkowitz R, Ghezzi F, et al. Maternal plasma and amniotic fluid 17 b-estradiol, progesterone and cortisol concentrations in women with successfully and unsuccessfully treated preterm labor. Arch Gynecol Obstet 1996;258:89–96.
Mazor M, Ghezzi F, Cohen J, et al. Maternal plasma and amniotic fluid dehydroepiandrosterone-sulfate concentrations in preterm labor and delivery. Arch Gynecol Obstet 1996;259:7–12.
Dudley DJ. Immunoendocrinology of preterm labor: The link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol 1999;180:S251–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors would like to thank Dr E. Economou for scientific advice, Dr E. Kostantellou for technical assistance and Drs G. Alexandrakis, P. Malligiannis, and J. Tziotis for collecting part of the material
Rights and permissions
About this article
Cite this article
Vrachnis, N., Malamitsi-Puchner, A., Samoli, E. et al. Elevated Mid-Trimester Amniotic Fluid ADAM-8 Concentrations as a Potential Risk Factor for Preterm Delivery. Reprod. Sci. 13, 186–190 (2006). https://doi.org/10.1016/j.jsgi.2006.01.003
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2006.01.003