Abstract
Objectives
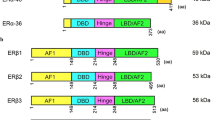
In different tissues, estrogens, selective estrogen receptor modulators (SERMs), and anti-estrogens exert different biologic activities. For the endometrium, estradiol and tamoxifen induce proliferation, and because of this, tamoxifen treatment of breast cancer patients results in a two- to sevenfold increased risk for development of endometrial cancer. Use of raloxifene, or the anti-estrogen ICI182780, does not result in such an increased risk. The objective of the current study was to generate and analyze gene expression profiles that reflect the transcriptional response of the human endometrium to estradiol, SERMs like tamoxifen and raloxifene, and anti-estrogens like ICI182780.
Methods
Transient transfections were peformed to analyze the transcriptional response of ECC-1 cells to estradiol, tamoxifen, raloxifene, and ICI182780. Subsequently, to reveal the molecular mechanism of action, gene expression profiles were generated and some of the observed regulated genes were confirmed by Northern blotting. Biostatistical methods were employed to analyze the expression profile results further, and amphiregulin effects on ECC-1 cell signaling were investigated using Northern and Western blotting, and 3 H-thymidine incorporation.
Results
Analysis of the profiles revealed that estradiol, tamoxifen, raloxifene, and ICI182780 influence the same biologic processes, but they do so via regulation of different sets of genes. Upon construction of a genetic network it was observed that the largest possible network centered on epidermal growth factor (EGF) receptor signaling. Furthermore, the EGF receptor ligand amphiregulin was differentially regulated by all four ligands. Next it was shown that amphiregulin indeed could stimulate EGF receptor signaling in ECC-1 cells. Based on these results, it was hypothesized that EGF receptor signaling could differentially be affected by estrogen, tamoxifen, raloxifene, and ICI182780 because these four compounds differentially regulate the EGF receptor ligand amphiregulin.
Conclusions
Regulation of amphiregulin coincides with the described in vivo effect of the four ligands on the endometrium. Therefore, it is possible that modulation of EGF receptor signaling is a significant player in estrogen-agonistic growth of the endometrium and needs to be investigated further.
Similar content being viewed by others
References
Akhmedkhanov A, Zeleniuch-Jacqoutte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: Review of the evidence and research perspectives. Ann N Y Acad Sci 2001;943:296–315.
Heikaus S, Winterhager E, Traub O, Grummer R. Responsiveness of endometrial genes Connexin26, Connexin43, C3 and clusterin to primary estrogen, selective estrogen receptor modulators, phyto- and xenoestrogens. J Mol Endocrinol 2002;29:239–249.
Buzdar A. The place of chemotherapy in the treatment of early breast cancer. Br J Cancer 1998;78 Suppl 4:16–20.
Powles TJ. Status of antiestrogen breast cancer prevention trials. Oncology (Huntingt) 1998;12 Suppl 5:28–31.
Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: prelimianry findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 1998;352:93–97.
Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 1992;326:852–856.
Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet 2000;356:881–887.
Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997;337:1641–1647.
Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporisis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637–645.
Jordan VC, Gapstur S, Morrow M. Selective estrogen receptor modulation and reduction in risk of breast cancer, osteoporosis, and coronary heart disease. J Natl Cancer Inst 2001;93:1449–1457.
Tobias JS. Endocrine approaches for the treatment of early and advanced breast cancer in postmenopausal women. Inst J Biochem Cell Biol 2004;36:2112–2119.
Osborne CK. Aromatase inhibitors in relation to other forms of endocrine therapy for breast cancer. Endocr Relat Cancer 1999;6:271–276.
Wardley AM. Fulvestrant: A review of its development, preclinical and clinical data. Int J Clin Pract 2002;56:305–309.
Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 1986;320:134–139.
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A 1996;93:5925–5930.
McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science 2002;296:1642–1644.
Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 2000;103:843–852.
Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science 2002;295:2465–2468.
Dardes RC, Schafer JM, Pearce ST, Osipo C, Chen B, Jordan VC. Regulation of estrogen target genes and growth by selective estrogen-receptor modulators in endometrial cancer cells. Gynecol Oncol 2002;85:498–506.
Satyaswaroop PG, Sivarajah A, Zaino RJ, Mortel R. hormonal control of growth of human endometrial carcinoma in the nude mouse model. In: Bresciane F, King RJB, Lippman M, Raynaud JP, eds. Progress in cancer research and therapy. New York: Raven Press, 1988:430–435.
Tremblay GB, Tremblay A, Copeland NG, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol 1997;11:353–365.
Fan JD, Wagner BL, McDonnell DP. Identification of the sequences within the human complement 3 promoter required for estrogen responsiveness provides insight into the mechanism f tamoxifen mixed agonist activity. Mol Endocrinol 1996;10:1605–1616.
Blok LJ, De Ruiter PE, Kuhne EC, et al. Progestogenic effects of tibolone on human endometrial cancer cells. J Clin Endocrinol Metab 2003;88:2327–2334.
Blok LJ, Grossmann ME, Perry JE, Tindall DJ. Characterization of an early growth response gene, which encodes a zinc finger transcription factor, potentially involved in cell cycle regulation. Mol Endocrinol 1995;9:1610–1620.
Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopaual women in a randomized breast cancer prevention trial. Lancet 1994;343:1318–1321.
Mourits MJ, De Vries EG, Willemse PH, Ten Hoor KA, Hollema H, Van der Zee AG. Tamoxifen treatment and gynecologic side effects: A review. Obstet Gynecol 2001;97:855–866.
Chang J, Powles TJ, Ashley SE, Iveson T, Gregory RK, Dowsett M. Variation in endometrial thickening in women with amenorrhea on tamoxifen. Breast Cancer Res Treat 1998;48:81–85.
Kian Tee M, Rogatsky I, Tzagarakis-Foster C, et al. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell 2004;15:1262–1272.
Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 2004;64:1522–1533.
Brzozowski AM, Pike AC, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997;389:753–758.
Feng W, Ribeiro RC, Wagner RL, et al. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 1998;280:1747–1749.
Shiau AK, Barstad D, Loria PM, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998;95:927–937.
Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol 2000;78:181–186.
Pfeiffer D, Spranger J, Al-Deiri M, et al. mRNA expression of ligands of the epidermal-growth-factor-receptor in the uterus. Int J Cancer 1997;72:581–586.
Kim JG, Vallet JL, Christenson RK. Molecular cloning and endometrial expression of porcine amphiregulin. Mol Reprod Dev 2003;65:366–372.
Vendrell JA, Magnino F, Danis E, et al. Estrogen regulation in human breast cancer cells of new downstream gene targets involved in estrogen metabolism, cell proliferation and cell transformation. J. Mol Endocrinol 2004;32:397–414.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors thank Wilfred Van IJcken from The Center for Biomics, Erasmus MC, Rotterdam, The Netherlands, for producing custom-made oligoarrays and for providing labeling protocols. In addition, we thank the Center for Biomics for facilitating BioAnalyzer RNA analysis, automated hybridization, array scanning, Imagene software, microarray normalization, and Rosetta Resolver software. Furthermore, we would like to thank Dr P. Giguere (Montreal, Canada) for the C3-luciferase construct, Dr D.P. McDonnell for the pS2-luciferase, and Dr M. Hibner (Scottsdale, AZ) for providing of Raloxifene hydrochloride.
Rights and permissions
About this article
Cite this article
Gielen, S.C.J.P., Burger, C.W., Kühne, L.C.M. et al. Analysis of Estrogen Agonism and Antagonism of Tamoxifen, Raloxifene, and ICI182780 in Endometrial Cancer Cells: A Putative Role for the Epidermal Growth Factor Receptor Ligand Amphiregulin. Reprod. Sci. 12, e55–e66 (2005). https://doi.org/10.1016/j.jsgi.2005.08.003
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2005.08.003