Abstract
Objective
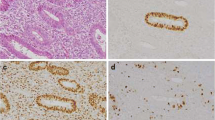
p53, MDM2, and p21Waf1 are oncoproteins that regulate the cell cycle. The purpose of this study was to examine the distribution of p53, MDM2, and p21Waf1 oncoprotein expression in endometriomas and in adenomyosis.
Methods
Tissue samples from 25 women with pathologically confirmed endometriomas and 3 1 women with pathologically confirmed adenomyosis were analyzed. Expression of p53, MDM2, and p21Waf1 oncoproteins was assessed by immunohistochemical nuclear staining.
Results
p53, MDM2, and p21Waf1 expression were detected in 20%, 60%, and 80%) of endometrioma tissue samples, respectively. All endometrioma tissue samples expressing p53 also tested positive for both MDM2 and p21Waf1. MDM2 expression was significantly higher in the proliferative than in the secretory phase of the cycle. In contrast, all 31 adenomyosis tissue samples were negative for p5 3, MDM2, and p21Waf1 expression.
Conclusion
The expression of p53, MDM2, and p2lWaf1 suggests a role for these oncoproteins in the regulation of endometrioma cell growth, but not in adenomyosis. (J Soc Gynecol Investig 2005; 12: 263— 6) Copyright © 2005 by the Society for Gynecologic Investigation.
Similar content being viewed by others
References
Harada T, Kaponis A, Iwabe T, et al. Apoptosis in human endometrium and endometriosis. Hum Reprod Update 2004;10:29–38.
Garcia-Velasco J, Arici A. Apoptosis and the pathogenesis of endometriosis. Semin Reprod Med 2003;21:165–72.
Witz CA. Pathogenesis of endometriosis. Gynecol Obstet Invest 2002;53:52–62.
Seli E, Berkkanoglu M, Arici A. Pathogenesis of endometriosis. Obstet Gynecol Clin North Am 2003;30:41–61.
Arici A, Matalliotakis I, Goumenou A, Koumantakis G, Vassiliadis S, Mahutte NG. Altered expression of interleukin-18 in the peritoneal fluid of women with endometriosis. Fertil Steril 2003;80:889–94.
Koumantakis E, Matalliotakis I, Neonaki M, Froudarakis G, Georgoulias V. Soluble serum interleukin-2 receptor, interleukin-6 and interleukin-1a in patients with endometriosis and in controls. Arch Gynecol Obstet 1994;255:107–12.
Vogelstein B, Kanzler KW. p53 function and dysfunction. Cell 1992;70:523–6.
Roemer K, Friedmann T. Mechanisms of action of the p53 tumor suppressor and prospects for cancer gene therapy by re-constitution of p53 function. Ann NY Acad Sei 1994; 716:265–80.
Cadwell C, Zambetti GP. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene 2001;277:15–30.
Chang F, Syijanen S, Syrjanen K. Implications of the p53 tumor-suppressor gene in clinical oncology. J Clin Oncol 1995;13:1009–22.
Wu X, Bayle JH, Olson D, Levme AJ. The p53-mdm-2 auto-regulatory feedback loop. Genes Dev 1993;7:1126–32.
Haupt Y, Maya R, Kazaz A, Oren M. MDM2 promotes the rapid degradation of p53. Nature 1997;387:296–9.
Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sei 1999;55:96–107.
El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993;75:817–25.
El-Deiry WS, Harper JW, O’connor PM, et al. WAFl/CIPl is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994;54:1169–74.
Valassiadou KE, Stefanaki K, Tzardi M, et al. Immunohistochemical expression of p53, bcl-2, MDM2 and wafl/p21 proteins in colorectal adenocarcinomas. Anticancer Res 1997;17:2571–6.
Plisiecka-Halasa J, Karpinska G, Szymanska T, et al. P21WAF1, P27KIP1, TP53 and C-MYC analysis in 204 ovarian carcinomas treated with platinum-based regimens. Ann Oncol 2003; 14:1078–85.
Milde-Langosch K, Hagen M, Bamberger Am, Loning T. Ex-pression and prognostic value of the cell-cycle regulatory pro-teins, Rb, pl6MTSl, p21WAFl, p27KIPl, cyclin E, and cyclin D2, in ovarian cancer. Int J Gynecol Pathol 2003;22:168–74.
Ferrandina G, Stoler A, Fagotti A, et al. p21WAFl/CIPl protein expression in primary ovarian cancer. Int J Oncol 2000;17:1231–5.
Taskin M, Lallas TA, Shevchuk M, Barber HR. P53 expression in adenomyosis in endometrial carcinoma patients. Gynecol On-col 1996;62:241–6.
Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril 1985;43:351–2.
Molitor JJ. Adenomyosis: A clinical and pathological appraisal. Am J Obstet Gynecol 1971;110:275–84.
Harada M, Suganuma N, Furuhashi M, et al. Detection of apoptosis in human endometriotic tissues. Mol Hum Reprod 1996;2:307–15.
Giannikaki E, Kouvidou C, Tzardi M, et al. p53 protein expression in breast carcinomas. Comparative study with the wild type p53 induced proteins MDM2 and p21/wafl. Anticancer Res 1997;17:2123–7.
Steinman RA, Hoffman B, Iro A, Guillouf C, Liebermann DA, el-Housei ME. Induction of p21 (WAF-1/CIP1) during differ-entiation. Oncogene 1994;9:3389–96.
Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R. MDM2 is a negative regulator of p21WAFl/CIPl, independent ofp53. J Biol Chem 2004;279:16000–6.
Bischoff FZ, Heard M, Simpson JL. Somatic DNA alterations in endometriosis: High frequency of chromosome 17 and p53 loss in late-stage endometriosis. J Reprod Immunol 2002;55:49–64.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by funds from the University of Crete.
The authors would like to thank Professor G. Delides, Chief of the Department of Pathology at the University of Crete, for his contribution to this work. We are also grateful for excellent technical support received from Elli Karidi.
Rights and permissions
About this article
Cite this article
Goumenou, A., Panayiotides, I., Mahutte, N.G. et al. Immunohistochemical Expression of p53, MDM2, and p21Waf1 Oncoproteins in Endometriomas But Not Adenomyosis. Reprod. Sci. 12, 263–266 (2005). https://doi.org/10.1016/j.jsgi.2005.01.028
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2005.01.028