Abstract
Objective
We have recently identified a novel putative spliced variant of the activating transcription factor 2 (ATF2) in the human myometrium during pregnancy and labor. This protein, termed ATF2-sm like full-length ATF2, acts as a potent trans activator of cyclic adenosine monophosphate response element (CRE)-containing promoter reporter genes. Similarly, employing microarray gene profiling in myometrial cells, we have shown ATF2-sm to affect the expression of several specific myometrial genes associated with regulating uterine activity during pregnancy and labor. At some point after conception this transcription factor becomes spatially expressed within the body of the uterus, with significantly higher levels detected in the upper (corpus) compared to the lower uterine segment. Because ATF2 species are the primary substrate for phosphorylation by the mitogen-activated protein kinases (MAPKs) p38 and ERK1/2, the purpose of the current investigation was to define the expression levels of these kinases in upper and lower segment myometrium during pregnancy and labor to see if they also correlated with expression of ATF2-sm.
Methods
Paired myometrial samples were collected from the upper (corpus) and lower uterine segments from term nonlaboring and spontaneously laboring women undergoing elective and emergency cesarean deliveries, respectively. Non-pregnant myometrial samples were collected from premenopausal women having hysterectomies for benign gynecologic disorders. The MAPKs p38 and ERK1/2 present in individual myometrial homogenates were resolved using sodium dodecyl sulfate polacrylamide gel electropheresis (SDS-PAGE) with subsequent Western blotting with specific antibodies and scanning densitometry. Expression of the individual MAPKs in myometrial tissues was confirmed in situ using immunohistochemistry.
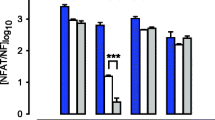
Results
In non-pregnant tissues, p38 and ERK1/2 expression was uniform throughout the uterus. In term pregnant nonlaboring and spontaneously laboring samples expression of p38 and ERK1 was significantly elevated in the upper uterine segment compared to the lower segment, respectively. In contrast, there was no difference in ERK2 expression.
Conclusion
The data from this study indicate that both p38 and ERK1 are spatially regulated in different uterine regions during pregnancy /labor and suggest that they may be involved in regulating the activity of ATF2 isoforms and their subsequent effects on myometrial function.
Similar content being viewed by others
References
Sparey C, Robson SC, Bailey J, Lyall F, Europe-Firmer GN. The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2 and Gsα proteins in the upper and lower segments of the human uterus during pregnancy and labour. J Clin Endocnnol Metab 1999;84:1705–10.
Pollard AJ, Sparey C, Robson SC, Krainer AR, Europe-Finner GN. Spatio-temporal expression of the trans acting splicing factors SF2/ASF and hnRNPA1/A1B in the myometrium of the pregnant human uterus: A molecular mechanism for regulating regional protein isoform expression in vivo. J Clin Endocrinol Metab 2000;85:1928–36.
Bailey J, Sparey C, Phillips RJ, et al. Expression of CREB, CREM and ATF2 cyclic AMP dependent transcription factors in the human myometrium during pregnancy and labour. Mol Hum Reprod 2000;6:648–60.
Bailey J, Phillips RJ, Pollard AJ, Gilmore K, Robson SC, Europe-Finner GN. Characterisation and functional analysis of CREM and ATF2 isoforms in the human myometrium during pregnancy and labour: Identification of a novel ATF2 species with potent transactivation properties. J Clin Endocrinol Metab 2002;87:1717–28.
Bailey J, Europe-Finner GN. Identification of human myometrial target genes of the c-Jun NH2-terminal kinase (JNK) pathway: the role of activating transcription factor 2 (ATF2) and a novel spliced isoform ATF2-small. J Mol Endocnnol 2005;34:19–35.
Hayakawa J, Depatie C, Ohmichi M, Mercola D. The activation of c-Jun NH2 terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2) dependent enhanced DNA repair. J Biol Chem 2003;278:20582–92.
Soorana SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein 1 factor in human myometrial cells. Mol Hum Reprod 2004;10:109–13.
Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1β: Involvement of p38 mitogen-activated protein kinase. J Physiol 1999;520:399–406.
Ohmichi M, Koike K, Kimura A, et al. Role of mitogen-activated protein kinase pathway in prostaglandin F2α- induced rat puerperal uterine contraction. Endocrinology 1997;138:3103–11.
Nohara A, Ohmichi M, Koike K, et al. The role of mitogen activated protein kinase in oxytocin induced contraction of uterine smooth muscle in pregnant rat. Biochem Biophys Res Comm 1996;229:938–44.
Kaiser GC, Yan F, Polk DB. Conversion of TNFa from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp Cell Res 1999;249:349–58.
Warn-Kramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem 1998;273:9188–96.
MacDougall MWJ, Europe-Finner GN, Robson SC. Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RIIα) protein kinase A holoenzyme. J Clin Endocnnol Metab 2003;88:2194–205.
Europe-Finner GN, Phaneuf S, Tolkovsky AM, Watson SP, Lopez Bernal A. Down-regulation of Gαs in human myometrium in term and preterm labour: A mechanism for parturition. J Clin Endocrinol Metab 1994;79:1835–9.
Oldenhof AD, Shynlova OP, Liu M, Langille BL, Lye SJ. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol 2002;283:0530–9.
Li Y, Je H-D, Malek S, Morgan KG. ERK 1/2-mediated phosphorylation of myometrial caldesmon during pregnancy and labour. Am J Physiol Regul Integr Comp Physiol 2003;284:R192–9.
Fuchs A, Fuchs F, Husslein P, Soloff M. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 1984;150:734–41.
Moonen P, Klok G, Keirse MJ. Distribution of prostaglandin enderoperoxide synthase and prostacyclin synthase in late pregnant uterus. Br J Obstet Gynaecol 1986;93:255–9.
Molnar M, Rigo J, Romero R, Hertelendy F. Oxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cells. Am J Obstet Gynecol 1999;181:42–9.
Strakova Z, Copeland JA, Lolait SJ, Soloff MS. ERK2 mediates oxytocin-stimulated PGE2 synthesis. Am J Physiol 1998;274:E634–41.
Yamazaki T, Komuro I, Yazaki Y. Signaling pathways for cardiac hypertrophy. Cell Signal 1998;10:693–8.
Douglas AJ, Clarke EW, Goldspink DF. Influence of mechanical stretch on growth and protein turnover of rat uterus. Am J Physiol 1988;254:E543–8.
Macphee DJ, Lye SJ. Focal adhesion signalling in the rat myometrium is abruptly terminated with the onset of labour. Endocrinology 2000;141:274–83.
Ruzycky AL. Effects of 17beta-estradiol and progesterone on mitogen-activated protein kinase expression and activity in rat uterine smooth muscle. Eur J Pharmacol 1996;300:247–54.
Author information
Authors and Affiliations
Corresponding author
Additional information
Work in this laboratory is funded by the Wellcome Trust and Action Medical Research.
Rights and permissions
About this article
Cite this article
Otun, H.A., MacDougall, M.W.J., Bailey, J. et al. Spatial and Temporal Expression of the Myometrial Mitogen-Activated Protein Kinases p38 and ERK1/2 in the Human Uterus During Pregnancy and Labor. Reprod. Sci. 12, 185–190 (2005). https://doi.org/10.1016/j.jsgi.2004.11.008
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2004.11.008