Abstract
Objective
We studied fetal endothelial function in a model of preeclampsia induced by Nω-nitro-l-arginine methylester (L-NAME) administration in pregnant rats.
Methods
Aortic segments from term fetuses and 2-day-old Wistar rats treated with L-NAME (0.5 mg/mL in drinking water) (fetuses from hypertensive rats, FH, and newborns from hypertensive rats, NH) and from untreated rats (fetuses from normotensive rats, FN, and newborns from normotensive rats, NN) were obtained. Endothelium-dependent and -independent relaxations were determined by the response to 1 μM acetylcholine (ACh) and 1 μM sodium nitroprusside (SNP), respectively, after precontraction with 3 μM prostaglandin F2α. The role of nitric oxide in ACh relaxation was assessed by incubation with 0.1 mM NG-monomethyl-l-arginine (L-NMMA) or 0.1 mM l-arginine (l-Arg). Precontraction with 50 mM potassium chloride assessed the role of hyperpolarizing mechanisms.
Results
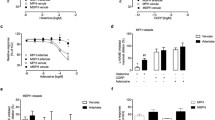
In FH, ACh-induced relaxation was reduced (FH 34.2 ± 4%, FN 45.8 ± 2%, P <.05), whereas that of SNP was enhanced (FH 68.4 ± 5%, FN 50.4 ± 4%, P <.05). l-Arg did not reverse the impairment of ACh relaxation. L-NMMA reduced ACh relaxation in FN but increased it in FH; this increase was abolished by potassium chloride precontraction and by 1 μM capsaicine, a calcitonin-gene related peptide inhibitor. The hyperpolarizing component of ACh relaxation was reduced in FH as compared with FN. By contrast, ACh relaxation was greater in NH than in NN, with the relative participation of nitric oxide and hyperpolarizing-related components being similar in both groups.
Conclusion
Fetal ACh relaxation was impaired in this preeclampsia-like model. This impairment is probably not exclusively an effect of L-NAME but could reflect endothelial dysfunction that disappears after birth.
Similar content being viewed by others
References
Naeye RL, Friedman EA. Causes of perinatal death associated with gestational hypertension and proteinuria. Am J Obstet Gynecol 1979;133:8–10.
Brazy JE, Grimm JK, Little VA. Neonatal manifestations of severe maternal hypertension ocurring before the thirty-sixth week of pregnancy. J Pediatr 1982;100:265–71.
Hartikainen AL, Aliharmi RH, Rantakallio PT. A cohort study of epidemiological associations and outcomes of pregnancies with hypertensive disorders. Hypertens Pregn 1998;17:31–41.
Martínez-Orgado J, Guardia-Nieto L, Cueto-Calvo E, Lozano-Maneiro L, Martínez MC, Torres-Martí J. Influencia del tipo de estado hipertensivo del embarazo en la aparición de complicaciones neonatales. Rev Esp Pediatr 1998;54:379–85.
Jacobson SL, Imhof R, Manning R, Mannion V, Little D, Rey E. The value of Doppler assessment of the uteroplacental circulation in predicting preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 1990;162:110–14.
Jouppila P, Kirkinen P. Blood velocity waveforms of the fetal aorta in normal and hypertensive pregnancies. Obstet Gynecol 1986;67:856–60.
Bush KD, O’Brien JM, Barton JR. The utility of umbilical artery Doppler investigation in women with the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol 2001;184:1087–9.
Martikainen AM, Heinonen KM, Saarikoski SV. The effect of the hypertension in pregnancy on fetal and neonatal condition. Int J Gynaecol Obstet 1989;30:213–20.
Zeeman GG, Dekker GA. Pathogenesis of preeclampsia: A hypothesis. Clin Obstet Gynecol 1992;35:317–37.
Roberts JM, Redman CWG. Preeclampsia: More than pregnancy-induced hypertension. Lancet 1993;341:1447–51.
Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol 1995;172:202–3.
Davidge ST, Signorella AP, Hubel CA, Lykins DL, Roberts JM. Distinct factors in plasma of preeclamptic women increase endothelial nitric oxide and prostacyclin. Hypertension 1996;28:758–64.
Davidge ST, Signorella AP, Lykins DL, Gilmour CH, Roberts JM. Evidence of endothelial activation and endothelial activators in cord blood of infants of preeclamptic women. Am J Obstet Gynecol 1996;175:1301–6.
McCarthy AL, Woolfson RG, Raju SK, Poston L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am J Obstet Gynecol 1993;168:1323–30.
Rutherford RAD, McCarthy A, Sullivan MHF, Elder MG, Polak JM, Wharton J. Nitric oxide synthase in human placenta and umbilical cord from normal, intrauterine growth-retarded and pre-eclamptic pregnancies. Br J Obstet Gynaecol 1995;116:3099–109.
Steinert JR, Wyatt AW, Poston L, Jacob R, Mann GE. Preeclampsia is associated with altered Ca2+ regulation and NO production in human fetal venous endothelial cells. FASEB J 2002;16:721–3.
Knock GA, Poston L. Bradykinin-mediated relaxation in isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am J Obstet Gynecol 1996;175:1668–74.
Pinto A, Sorrentino R, Sorrentino P, Guerritore T, Miranda L, Biondi A. Endothelial-derived relaxing factor released by endothelial cells of human umbilical vessel and its impairment in pregnancy-induced hypertension. Am J Obstet Gynecol 1991;164:507–13.
Arias F. Accuracy of the middle-cerebral-to-umbilical-artery resistance index ratio in the prediction of neonatal outcome in patients at high risk for fetal and neonatal complications. Am J Obstet Gynecol 1994;171:1541–5.
Molnár M, Sütö T, Tóth T, Hertelendy F. Prolonged blockade of nitric oxide synthase in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol 1994;170:1458–66.
Martínez-Orgado J, González R, Tovar S, Marín J, Salaices M, Alonso MJ. Administration of Nω-L-arginine methylester (L-NAME) impairs endothelium-dependent relaxation in gravid but not in non-gravid rats. J Soc Gynecol Investig 2003;2:74–81.
Martínez-Orgado J, González R, Alonso MJ, Marín J. Nitric oxide-dependent and -independent mechanisms in the relaxation elicited by acetylcholine in fetal rat aorta. Life Sci 1999;64:269–77.
Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991;43:109–42.
Hishikawa K, Kakaki T, Suzuki H, Kato R, Saruta T. Role of L-arginine: Nitric oxide pathway in hypertension. J Hypertens 1993;11:639–45.
Marin J, Rodriguez-Martinez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol Ther 1997;75:111–34.
Zhao H, Shimokawa H, Uragami-Harasawa L, Igarashi H, Takeshita A. Long-term vascular effects of Nω-nitro-L-arginine methyl ester are not solely mediated by inhibition of endothelial nitric oxide synthesis in the rat mesenteric artery. J Cardiovasc Pharmacol 1999;33:554–66.
Corriu C, Feletou M, Puybasset L, Bea ML, Berdeaux A, Vanhoutte PM. Endothelium-dependent hyperpolarization in isolated arteries taken from animals treated with NO-synthase inhibitors. J Cardiovasc Pharmacol 1998;32:944–50.
Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 1996;94:3341–7.
Maeso R, Navarro-Cid J, Rodrigo E, Ruilope LM, Cachofeiro V, Lahera V. Effects of antihypertensive therapy on factors mediating endothelium-dependent relaxation in rats treated chronically with L-NAME. J Hypertens 1999;17:221–7.
Gangula PR, Supowit SC, Wimalawansa SJ, et al. Calcitonin gene-related peptide is a depressor in NG-nitro-L-arginine methyl ester-induced hypertension during pregnancy. Hypertension 1997;29:248–53.
Voelker CA, Miller MJ, Zhang XJ, Eloby-Childress S, Clark DA, Pierce MJ. Perinatal nitric oxide synthase inhibition retards neonatal growth by inducing hypertrophic pyloric stenosis in rats. Pediatr Res 1995;35:768–74.
Pearce WJ, Longo LD. Developmental aspects of endothelial function. Semin Perinatol 1991;15:40–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from D.G.I.C.Y.T. (BXX-2000-0153), and FISS (P1021540).
The authors thank the vererinarian Dr. María del Carmen Fernández-Criado for the care of animals, Fernando Nuñez and Lourdes Hernández for their excellent technical assistance, and Ms. Carol Warren for her linguistic assistance.
Rights and permissions
About this article
Cite this article
Martínez-Orgado, J., González, R., Alonso, M.J. et al. Impairment of Fetal Endothelium-Dependent Relaxation in a Rat Model of Preeclampsia by Chronic Nitric Oxide Synthase Inhibition. Reprod. Sci. 11, 82–88 (2004). https://doi.org/10.1016/j.jsgi.2003.08.003
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2003.08.003