Abstract
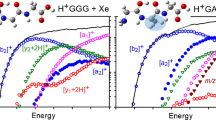
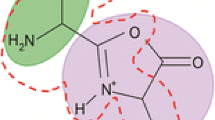
Extensive 15N labeling and multiple-stage tandem mass spectrometry were used to investigate the fragmentation pathways of the model peptide FGGFL during low-energy collision-induced-dissociation (CID) in an ion-trap mass spectrometer. Of particular interest was formation of a 4 from b 4 and a*4 (a 4-NH3) from a 4 ions correspondingly, and apparent rearrangement and scrambling of peptide sequence during CID. It is suggested that the original FGGFoxa b 4 structure undergoes b-type scrambling to form GGFFoxa. These two isomers fragment further by elimination of CO and 14NH3 or 15NH3 to form the corresponding a 4and a*4 isomers, respectively. For (15N-F)GGFL and FGG(15N-F)L the a*4 ion population appears as two distinct peaks separated by 1 mass unit. These two peaks could be separated and fragmented individually in subsequent CID stages to provide a useful tool for exploration of potential mechanisms along the a 4 → a*4 pathway reported previously in the literature (Vachet et al. J. Am. Chem. Soc. 1997, 119, 5481, and Cooper et al. J. Am. Soc. Mass Spectrom. 2006, 17, 1654). These mechanisms result in formally the same a*4 structures but differ in the position of the expelled nitrogen atom. Detailed analysis of the observed fragmentation patterns for the separated light and heavy a*4 ion fractions of (15N-F)GGFL indicates that the mechanism proposed by Cooper et al. is consistent with the experimental findings, while the mechanism proposed by Vachet et al. cannot account for the labeling data. In addition, a new rearrangement pathway is presented for a 4*-CO ions that effectively transfers the former C-terminal amino acid residue to the N-terminus.
Article PDF
Similar content being viewed by others
References
Hunt, D. F.; Yates, J. R. III; Shabanonowitz, J.; Winston, S.; Hauer, C. R. Protein Sequencing by Tandem Mass Spectrometry. Proc. Natl. Acad. Sci. U.S.A. 1986, 83, 6233–6237.
Biemann, K. Contributions of Mass Spectrometry to Peptide and Protein Structure. Biomed. Environ. Mass Spectrom. 1988, 16, 99–111.
Paizs, B.; Suhai, S. Fragmentation Pathways of Protonated Peptides. Mass Spectrom. Rev. 2004, 24, 508–548.
Steen, H.; Mann, M. The abc’s and the xyz’s of Peptide Sequencing. Nat. Rev. Mol. Cell. Biol. 2004, 5, 699–711.
Nesvizhskii, A. E.; Vitek, O.; Aebersold, R. Analysis and Validation of Proteomic Data Generated by Tandem Mass Spectrometry. Nat. Methods 2007, 4, 787–797.
Roepstorff, P.; Fohlmann, J. Proposal for a Common Nomenclature for Sequence Ions in Mass Spectra of Peptides. J. Biomed. Mass Spectrom. 1984, 11601.
Biemann, K. Contributions of Mass Spectrometry to Peptide and Protein Structure. Biomed. Environ. Mass Spectrom. 1988, 16, 99.
Dongré, A. R.; Jones, J. L.; Somogyi, Á.; Wysocki, V. H. Influence of Peptide Composition, Gas-Phase Basicity and Chemical Modification on Fragmentation Efficiency: Evidence for the Mobile Proton Model. J. Am. Chem. Soc. 1996, 118, 8365–8374.
Wysocki, V. H.; Tsaprailis, G.; Smith, L. L.; Breci, L. A. Mobile and Localized Protons: A Framework for Understanding Peptide Dissociation. J. Mass Spectrom. 2000, 35, 1399–1406.
Yalcin, T.; Csizmadia, I. G.; Peterson, M. B.; Harrison, A. Why are b-Ions Stable Species in Peptide Spectra? J. Am. Soc. Mass Spectrom. 1996, 7, 233–242.
Paizs, B.; Lendvay, G.; Vékey, K.; Suhai, S. Formation of b +2 Ions from Protonated Peptides: An ab Initio Study. Rapid Commun. Mass Spectrom. 1999, 13, 525–533.
Paizs, B.; Suhai, S. Towards Understanding the Tandem Mass Spectra of Protonated Oligopeptides: 1: Mechanism of Amide Bond Cleavage. J. Am. Soc. Mass Spectrom. 2004, 15, 103–113.
Paizs, B.; Suhai, S. Combined Quantum Chemical and RRKM Modeling of the Main Fragmentation Pathways of Protonated GGG: II. Formation of b2, y1, and y2 ions. Rapid Commun. Mass Spectrom. 2002, 16, 375–389.
Polce, M. J.; Ren, D.; Wesdemiotis, C. Dissociation of the Peptide Bond in Protonated Peptides. J. Mass Spectrom. 2000, 35(12), 1391–1398.
Cordero, M. M.; Houser, J. J.; Wesdemiotis, C. The Neutral Products Formed During Backbone Fragmentations of Protonated Peptides in Tandem Mass Spectrometry. Anal. Chem. 1993, 65, 1594–1601.
Nold, M. J.; Wesdemiotis, C.; Yalcin, T.; Harrison, A. G. Amide Bond Dissociation in protonated Peptides: Structures of the N-terminal Ionic and Neutral Fragments. Int. J. Mass Spectrom. Ion Processes 1997, 164, 137–153.
Yalcin, T.; Khouw, C.; Csizmadia, I. G.; Peterson, M. R.; Harrison, A.G. The Structure and Fragmentation of b n (n ⩾ 3) Ions in Peptide Spectra. J. Am. Soc. Mass Spectrom. 1995, 6, 1165–1174.
Polfer, N. C.; Oomens, J.; Suhai, S.; Paizs, B. Spectroscopic and Theoretical Evidence for Oxazolone Ring Formation in Collision Induced Dissociation of Peptides. J. Am. Chem. Soc. 2005, 127, 17154–17155.
Polfer, N. C.; Oomens, J.; Suhai, S.; Paizs, B. Infrared Spectroscopy and Theoretical Studies on Gas-Phase Protonated Leu-enkephalin and Its Fragments: Direct Experimental Evidence for the Mobile Proton. J. Am. Chem. Soc. 2007, 129, 5887–5897.
Harrison, A. G.; Young, A. B.; Bleiholder, B.; Suhai, S.; Paizs, B. Scrambling of Sequence Information in Collision-Induced Dissociation of Peptides. J. Am. Chem. Soc. 2006, 128, 10364–10365.
Tang, X. -J.; Thibault, P.; Boyd, R. K. Fragmentation Reactions of Multiply-protonated Peptides and Implications for Sequencing by Tandem Mass-spectrometry with Low-Energy Collision-induced Dissociation. Anal. Chem. 1993, 65, 2824–2834.
Riba-Garcia, I.; Giles, K.; Bateman, R. H.; Gaskell, S. J. Evidence for Structural Variants of a- and b-Type Peptide Fragment Ions Using Combined Ion Mobility/Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 609–613.
Farrugia, J. M.; O’Hair, R. A. J.; Reid, G. E. Do All b2 Ions Have Oxazolone Structures?: Multistage Mass Spectrometry and Ab Initio Studies on Protonated N-Acyl Amino Acid Methyl Ester Model Systems. Int. J. Mass Spectrom. Ion Processes 2001, 210/211, 71–87.
Gu, C.; Tsaprailis, G.; Breci, L.; Wysocki, V. H. Selective Gas-Phase Cleavage at the Peptide Bond C-Terminal to Aspartic Acid in Fixed-Charge Derivatives of Asp-Containing Peptides. Anal. Chem. 2000, 72, 5804–5813.
Tsaprailis, G.; Nair, H.; Somogyi, A.; Wysocki, V. H.; Zhong, W.; Futrell, J. H.; Summerfield, S. G.; Gaskell, S. J. Influence of Secondary Structure on the Fragmentation of Protonated Peptides. J. Am. Chem. Soc. 1999, 121, 5142–5154.
Farrugia, J. M.; Taverner, T.; O’Hair, R. A. J. Side-Chain Involvement in the Fragmentation Reactions of the Protonated Methyl Esters of Histidine and Its Peptides. 2001, 209, 99–112.
Savitski, M. M.; Falth, M.; Eva Fung, Y. M.; Adams, C. M.; Zubarev, R. A Bifurcating Fragmentation Behavior of Gas-Phase Tryptic Peptide Dications in Collisional Activation. J. Am. Soc. Mass Spectrom. (2008), doi. 10.1016/j.jasms.2008.08.003.
Vachet, R. W.; Ray, K. L.; Glish, G. L. Origin of Product Ions in the MS/MS Spectra of Peptides in a Quadrupole Ion Trap. J. Am. Soc. Mass Spectrom. 1998, 9, 341–344.
Paizs, B.; Szlavik, Z.; Lendvay, G.; Vékey, K.; Suhai, S. Formation of a +2 ions of protonated peptides: An Ab Initio Study. Rapid Commun. Mass Spectrom. 2000, 14, 746–755.
Vachet, R. W.; Bishop, B. M.; Erickson, B. W.; Glish, G. L. Novel Peptide Dissociation: Gas-Phase Intramolecular Rearrangement of Internal Amino Acid Residues. J. Am. Chem. Soc. 1997, 119, 5481–5488.
Polfer, N. C.; Bohrer, B. C.; Plasencia, M. D.; Paizs, B.; Clemmer, D. E. On the Dynamics of Fragment Isomerization in Collision-Induced Dissociation of Peptides. J. Phys. Chem. A 2008, 112, 1286–1293.
Bleiholder, C.; Osburn, S.; Williams, T. D.; Suhai, S.; Van Stipdonk, M.; Harrison, A. G.; Paizs, B. Sequence Scrambling Fragmentation Pathways of Protonated Peptides, unpublished.
Fmoc Solid Phase Peptide Synthesis—A Practical Approach; Chan, W. C.; White, P. D.; Eds; Oxford University Press: New York, 2000.
Barr, J. M.; Van Stipdonk, M. J. Multi-Stage Tandem Mass Spectrometry of Metal Cationized Leucine Enkephalin and Leucine Enkephalin Amide. Rapid Commun. Mass Spectrom. 2002, 16, 566–578.
Paizs, B.; Suhai, S. Combined Quantum Chemical and RRKM Modeling of the Main Fragmentation Pathways of Protonated GGG: II. Formation of b2, y1, and y2 ions. Rapid Commun. Mass Spectrom. 2002, 16, 375–389.
Case, D. A.; Pearlman, D. A.; Caldwell, J. W.; Cheatham, T. E., III; Ross, W. S.; Simmerling, C. L.; Darden, T. A.; Merz, K. M.; Stanton, R. V.; Cheng, A. L.; Vincent, J. J.; Crowley, M.; Tsui, V.; Radmer, R. J.; Duan, Y.; Pitera, J.; Massova, I. G.; Seibel, G. L.; Singh, U. C.; Weiner, P. K.; Kollmann, P. A. AMBER 99, University of California: San Francisco, 1999.
Gaussian 03, Revision C.02, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A. III; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; and Pople, J. A.; Gaussian, Inc., Wallingford CT, 2004.
Harrison, A. G.; Young, A. B. Fragmentation of Protonated Oligoalanines: Amide Bond Cleavage and Beyond. J. Am. Soc. Mass Spectrom. 2004, 15, 1810–1819.
Cooper, T.; Talaty, E.; Grove, J.; Suhai, S.; Paizs, B.; Van Stipdonk, M. Isotope Labeling and Theoretical Study of the Formation of a*3 Ions from Protonated Tetraglycine. J. Am. Soc. Mass Spectrom. 2006, 17, 1654–1664.
Bythell, B. J.; Barofsky, D. F.; Pingitore, F.; Wang, P.; Wesdemiotis, C.; Paizs, B. Backbone Cleavages and Sequential Loss of Carbon Monoxide and Ammonia from Protonated AGG: A Combined Tandem Mass Spectrometry, Isotope Labeling, and Theoretical Study. J. Am. Soc. Mass Spectrom. 2007, 18, 1291–1303.
Kinser, R. D.; Ridge, D. P.; Hvistendahl, G.; Rasmussen, B.; Uggerud, E. The Unimolecular Chemistry of Protonated Glycinamide and the Proton Affinity of Glycinamide Mass Spectrometric Experiments and Theoretical Model. Chem. Eur. J. 1996, 2, 1143–1149.
Allen, J. M.; Black, D. M.; Johnson, J. S.; Glish, G. L.; Bythell, B. J.; Paizs, B. Why Do b3 Ions not Form a3 Ions? Unpublished.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published online August 19, 2008
Rights and permissions
About this article
Cite this article
Bythell, B.J., Molesworth, S., Osburn, S. et al. Structure and reactivity of a n and a* n peptide fragments investigated using isotope labeling, tandem mass spectrometry, and density functional theory calculations. J Am Soc Mass Spectrom 19, 1788–1798 (2008). https://doi.org/10.1016/j.jasms.2008.08.010
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/j.jasms.2008.08.010