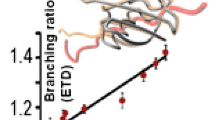
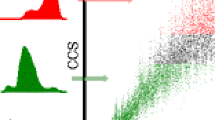
Abstract
Effects of protein conformation on electron capture dissociation (ECD) were investigated using high-field asymmetric waveform ion mobility spectrometry (FAIMS) and Fourier-transform ion cyclotron resonance mass spectrometry. Under the conditions of these experiments, the electron capture efficiency of ubiquitin 6+ formed from three different solution compositions differs significantly, ranging from 51 ± 7% for ions formed from an acidified water/methanol solution to 88 ± 2% for ions formed from a buffered aqueous solution. This result clearly indicates that these protein ions retain a memory of their solution-phase structure and that conformational differences can be probed in an ECD experiment. Multiple conformers for the 7+ and 8+ charge states of ubiquitin were separated using FAIMS. ECD spectra of conformer selected ions of the same charge states differ both in electron capture efficiency and in the fragment ion intensities. Conformers of a given charge state that have smaller collisional cross sections can have either a larger or smaller electron capture efficiency. A greater electron capture efficiency was observed for ubiquitin 6+ that has the same collisional cross section as one ubiquitin 7+ conformer, despite the lower charge state. These results indicate that the shape of the molecule can have a greater effect on electron capture efficiency than either collisional cross section or charge state alone. The cleavage locations of different conformers of a given charge state were the same indicating that the presence of different conformers in the gas phase is not due to difference in where charges are located, but rather reflect conformational differences most likely originating from solution. Small neutral losses observed from the singly- and doubly-reduced ubiquitin 6+ do not show a temperature dependence to their formation, consistent with these ions being formed by nonergodic processes.
Article PDF
Similar content being viewed by others
References
Zubarev, R. A.; Kelleher, N. L.; McLafferty, F. W. Electron Capture Dissociation of Multiply Charged Protein Cations: A Nonergodic Process. J. Am. Chem. Soc. 1998, 120, 3265–3266.
Zubarev, R. A. Reactions of Polypeptide Ions with Electrons in the Gas Phase. Mass Spectrom. Rev. 2003, 22, 57–77.
Cooper, H. J.; Hakansson, K.; Marshall, A. G. The Role of Electron Capture Dissociation in Biomolecular Analysis. Mass Spectrom. Rev. 2005, 24, 201–222.
Hakansson, K.; Cooper, H. J.; Emmett, M. R.; Costello, C. E.; Marshall, A. G.; Nilsson, C. L. Electron Capture Dissociation and Infrared Multiphoton Dissociation MS/MS of an N-Glycosylated Tryptic Peptide to Yield Complementary Sequence Information. Anal. Chem. 2001, 73, 4530–4536.
Mirgorodskaya, E.; Roepstorff, P.; Zubarev, R. A. Localization of O-Glycosylation Sites in Peptides by Electron Capture Dissociation in a Fourier Transform Mass Spectrometer. Anal. Chem. 1999, 71, 4431–4436.
Ge, Y.; Lawhorn, B. G.; El-Naggar, M.; Strauss, E.; Park, J. H.; Begley, T. P.; McLafferty, F. W. Top Down Characterization of Larger Proteins (45 kDa) by Electron Capture Dissociation Mass Spectrometry. J. Am. Chem. Soc. 2002, 124, 672–678.
Sze, S. K.; Ge, Y.; Oh, H.; McLafferty, F. W. Top-Down Mass Spectrometry of a 29-kDa Protein for Characterization of Any Posttranslational Modification to Within One Residue. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 1774–1779.
Kelleher, R. L.; Zubarev, R. A.; Bush, K.; Furie, B.; Furie, B. C.; McLafferty, F. W.; Walsh, C. T. Localization of Labile Posttranslational Modifications by Electron Capture Dissociation: The Case of γ-Carboxyglutamic Acid. Anal. Chem. 1999, 71, 4250–4253.
Stensballe, A.; Jensen, O. N.; Olsen, J. V.; Haselmann, K. F.; Zubarev, R. A. Electron Capture Dissociation of Singly and Multiply Phosphorylated Peptides. Rapid Commun. Mass Spectrom. 2000, 14, 1793–1800.
Ge, Y.; El-Naggar, M.; Sze, S. K.; Bin Oh, H.; Begley, T. P.; McLafferty, F. W.; Boshoff, H.; Barry, C. E. Top Down Characterization of Secreted Proteins from Mycobacterium tuberculosis by Electron Capture Dissociation Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 253–261.
Meng, F. Y.; Cargile, B. J.; Miller, L. M.; Forbes, A. J.; Johnson, J. R.; Kelleher, N. L. Informatics and Multiplexing of Intact Protein Identification in Bacteria and the Archaea. Nat. Biotechnol. 2001, 19, 952–957.
Leymarie, N.; Berg, E. A.; McComb, M. E.; O’Connor, P. B.; Grogan, J.; Oppenheim, F. G.; Costello, C. E. Tandem Mass Spectrometry for Structural Characterization of Proline-Rich Proteins: Application to Salivary PRP-3. Anal. Chem. 2002, 74, 4124–4132.
Breuker, K.; Oh, H. B.; Horn, D. M.; Cerda, B. A.; McLafferty, F. W. Detailed Unfolding and Folding of Gaseous Ubiquitin Ions Characterized by Electron Capture Dissociation. J. Am. Chem. Soc. 2002, 124, 6407–6420.
Syka, J. E. P.; Coon, J. J.; Schroeder, M. J.; Shabanowitz, J.; Hunt, D. F. Peptide and Protein Sequence Analysis by Electron Transfer Dissociation Mass Spectrometry. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 9528–9533.
Pitteri, S. J.; Chrisman, P. A.; Hogan, J. M.; McLuckey, S. A. Electron Transfer Ion/Ion Reactions in a Three-Dimensional Quadrupole Ion Trap: Reactions of Doubly and Triply Protonated Peptides with SO −±2 . Anal. Chem. 2005, 77, 1831–1839.
Coon, J. J.; Shabanowitz, J.; Hunt, D. F.; Syka, J. E. P. Electron Transfer Dissociation of Peptide Anions. J. Am. Soc. Mass Spectrom. 2005, 16, 880–882.
Chrisman, P. A.; Pitteri, S. J.; Hogan, J. M.; McLuckey, S. A. SO −±2 : Electron Transfer Ion/Ion Reactions with Disulfide Linked Polypeptide Ions. J. Am. Soc. Mass Spectrom. 2005, 16, 1020–1030.
Nielsen, M. L.; Budnik, B. A.; Haselmann, K. F.; Zubarev, R. A. Tandem MALDI/El Ionization for Tandem Fourier Transform Ion Cyclotron Resonance Mass Spectrometry of Polypeptides. Int. J. Mass Spectrom. 2003, 226, 181–187.
Hvelplund, P.; Liu, B.; Nielsen, S. B.; Tomita, S.; Cederquist, H.; Jensen, J.; Schmidt, H. T.; Zettergren, H. Electron Capture and Loss by Protonated Peptides and Proteins in Collisions with C60 and Na. Eur. Phys. J. D. 2003, 22, 75–79.
Hvelplund, P.; Liu, B.; Nielsen, S. B.; Tomita, S. Electron Capture Induced Dissociation of Peptide Dications. Int. J. Mass Spectrom. 2003, 225, 83–87.
Mormann, M.; Peter-Katalinic, J. Improvement of Electron Capture Efficiency by Resonant Excitation. Rapid Commun. Mass Spectrom. 2003, 17, 2208–2214.
Zubarev, R. A.; Horn, D. M.; Fridriksson, E. K.; Kelleher, N. L.; Kruger, N. A.; Lewis, M. A.; Carpenter, B. K.; McLafferty, F. W. Electron Capture Dissociation for Structural Characterization of Multiply Charged Protein Cations. Anal. Chem. 2000, 72, 563–573.
Iavarone, A. T.; Paech, K.; Williams, E. R. Effects of Charge State and Cationizing Agent on the Electron Capture Dissociation of a Peptide. Anal. Chem. 2004, 76, 2231–2238.
Zubarev, R. A.; Haselmann, K. F.; Budnik, B.; Kjeldsen, F.; Jensen, F. Towards an Understanding of the Mechanism of Electron-Capture Dissociation: A Historical Perspective and Modern Ideas. Eur. J. Mass Spectrom. 2002, 8, 337–349.
Suckau, D.; Shi, Y.; Beu, S. C.; Senko, M. W.; Quinn, J. P.; Wampler, F. M.; McLafferty, F. W. Coexisting Stable Conformations of Gaseous Protein Ions. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 790–793.
Freitas, M. A.; Hendrickson, C. L.; Emmett, M. R.; Marshall, A. G. Gas-Phase Bovine Ubiquitin Cation Conformations Resolved by Gas-Phase Hydrogen/Deuterium Exchange Rate and Extent. Int. J. Mass Spectrom. 1999, 185/186/187, 565–575.
Winger, B. E.; Light-Wahl, K. J.; Rockwood, A. L.; Smith, R. D. Probing Qualitative Conformation Differences of Multiply Protonated Gas-Phase Proteins via H/D Isotopic Exchange with D2O. J. Am. Chem. Soc. 1992, 114, 5897–5898.
Jarrold, M. F. Peptides and Proteins in the Vapor Phase. Annu. Rev. Phys. Chem. 2000, 51, 179–207.
Clemmer, D. E.; Hudgins, R. R.; Jarrold, M. F. Naked Protein Conformations—Cytochrome c in the Gas Phase. J. Am. Chem. Soc. 1995, 117, 10141–10142.
Myung, S.; Badman, E. R.; Lee, Y. J.; Clemmer, D. E. Structural Transitions of Electrosprayed Ubiquitin Ions Stored in an Ion Trap over ∼10 ms to 30 s. J. Phys. Chem. A. 2002, 106, 9976–9982.
Loo, R. R. O.; Loo, J. A.; Udseth, H. R.; Fulton, J. L.; Smith, R. D. Protein Structural Effects in Gas-Phase Ion Molecule Reactions with Diethylamine. Rapid Commun. Mass Spectrom. 1992, 6, 159–165.
Gross, D. S.; Schnier, P. D.; Rodriguez-Cruz, S. E.; Fagerquist, C. K.; Williams, E. R. Conformations and Folding of Lysozyme Ions in Vacuo. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 3143–3148.
Valentine, S. J.; Counterman, A. E.; Clemmer, D. E. Conformer-Dependent Proton-Transfer Reactions of Ubiquitin Ions. J. Am. Soc. Mass Spectrom. 1997, 8, 954–961.
Reimann, C. T.; Sullivan, P. A.; Axelsson, J.; Quist, A. P.; Altmann, S.; Roepstorff, P.; Velazquez, I.; Tapia, O. Conformation of Highly-Charged Gas-Phase Lysozyme Revealed by Energetic Surface Imprinting. J. Am. Chem. Soc. 1998, 120, 7608–7616.
Badman, E. R.; Hoaglund-Hyzer, C. S.; Clemmer, D. E Dissociation of Different Conformations of Ubiquitin Ions. J. Am. Soc. Mass Spectrom. 2002, 13, 719–723.
Purves, R. W.; Barnett, D. A.; Guevremont, R. Separation of Protein Conformers using Electrospray-High Field Asymmetric Waveform Ion Mobility Spectrometry-Mass Spectrometry. Int. J. Mass Spectrom. 1999, 197, 163–177.
Guevremont, R. High-Field Asymmetric Waveform Ion Mobility Spectrometry: A New Tool for Mass Spectrometry. J. Chromatogr. A. 2004, 1058, 3–19.
Robinson, E. W.; Williams, E. R. Multidimensional Separations of Ubiquitin Conformers in the Gas Phase: Relating Ion Cross Sections to H/D Exchange Measurements. J. Am. Soc. Mass Spectrom. 2005, 16, 1427–1437.
Robinson, E. W.; Garcia, D. E.; Leib, R. D.; Williams, E. R. Enhanced Mixture Analysis of Poly(Ethylene Glycol) Using High-Field Asymmetric Waveform Ion Mobility Spectrometry Combined with Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem. 2006, 78, 2190–2198.
Purves, R. W.; Barnett, D. A.; Ells, B.; Guevremont, R. Investigation of Bovine Ubiquitin Conformers Separated by High-Field Asymmetric Waveform Ion Mobility Spectrometry: Cross Section Measurements Using Energy-Loss Experiments with a Triple Quadrupole Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2000, 11, 738–745.
Viehland, L. A.; Guevremont, R.; Purves, R. W.; Barnett, D. A. Comparison of High-Field Ion Mobility Obtained from Drift Tubes and a FAIMS Apparatus. Int. J. Mass Spectrom. 2000, 197, 123–130.
Jurchen, J. C.; Williams, E. R. Origin of Asymmetric Charge Partitioning in the Dissociation of Gas-Phase Protein Homodimers. J. Am. Chem. Soc. 2003, 125, 2817–2826.
Chowdhury, S. K.; Katta, V.; Chait, B. T. Probing Conformational Changes in Proteins by Mass Spectrometry. J. Am. Chem. Soc. 1990, 112, 9013–9015.
Loo, J. A.; Loo, R. R. O.; Udseth, H. R.; Edmonds, C. G.; Smith, R. D. Solvent-Induced Conformational-Changes of Polypeptides Probed by Electrospray-Ionization Mass-Spectrometry. Rapid Commun. Mass Spectrom. 1991, 5, 101–105.
Li, J.; Taraszka, J. A.; Counterman, A. E.; Clemmer, D. E. Influence of Solvent Composition and Capillary Temperature on the Conformations of Electrosprayed Ions: Unfolding of Compact Ubiquitin Conformers from Pseudonative and Denatured Solutions. Int. J. Mass Spectrom. 1999, 185/186/187, 37–47.
Eyles, S. J.; Kaltashov, I. A. Methods to Study Protein Dynamics and Folding by Mass Spectrometry. Methods. 2004, 34, 88–99.
Mohimen, A.; Dobo, A.; Hoerner, J. K.; Kaltashov, I. A. A Chemometric Approach to Detection and Characterization of Multiple Protein Conformers in Solution Using Electrospray Ionization Mass Spectrometry. Anal. Chem. 2003, 75, 4139–4147.
Iavarone, A. T.; Jurchen, J. C.; Williams, E. R. Effects of Solvent on the Maximum Charge State and Charge State Distribution of Protein Ions Produced by Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2000, 11, 976–985.
Iavarone, A. T.; Williams, E. R. Mechanism of Charging and Supercharging Molecules in Electrospray Ionization. J. Am. Chem. Soc. 2003, 125, 2319–2327.
Cerda, B. A.; Breuker, K.; Horn, D. M.; McLafferty, F. W. Charge/Radical Site Initiation Versus Coulombic Repulsion for Cleavage of Multiply Charged Ions: Charge Solvation in Poly(Alkene Glycol) Ions. J. Am. Soc. Mass Spectrom. 2001, 12, 565–570.
Budnik, B. A.; Nielsen, M. L.; Olsen, J. V.; Haselmann, K. F.; Horth, P.; Haehnel, W.; Zubarev, R. A. Can Relative Cleavage Frequencies in Peptides Provide Additional Sequence Information?. Int. J. Mass Spectrom. 2002, 219, 283–294.
Zubarev, R. A.; Kruger, N. A.; Fridriksson, E. K.; Lewis, M. A.; Horn, D. M.; Carpenter, B. K.; McLafferty, F. W. Electron Capture Dissociation of Gaseous Multiply-Charged Proteins is Favored at Disulfide Bonds and Other Sites of High Hydrogen Atom Affinity. J. Am. Chem. Soc. 1999, 121, 2857–2862.
Breuker, K.; Oh, H. B.; Cerda, B. A.; Horn, D. M.; McLafferty, F. W. Hydrogen Atom Loss in Electron-Capture Dissociation: A Fourier Transform-Ion Cyclotron Resonance Study with Single Isotopomeric Ubiquitin Ions. Eur. J. Mass Spectrom. 2002, 8, 177–180.
Jockusch, R. A.; Schnier, P. D.; Price, W. D.; Strittmatter, E. F.; Demirev, P. A.; Williams, E. R. Effects of Charge State on Fragmentation Pathways, Dynamics, and Activation Energies of Ubiquitin Ions Measured by Blackbody Infrared Radiative Dissociation. Anal. Chem. 1997, 69, 1119–1126.
Price, W. D.; Schnier, P. D.; Jockusch, R. A.; Strittmatter, E. F.; Williams, E. R. Unimolecular Reaction Kinetics in the High-Pressure Limit Without Collisions. J. Am. Chem. Soc. 1996, 43, 10640–10644.
The internal energy of ubiquitin in these studies was estimated by assuming a Boltzmann contribution of internal energy from each vibrational mode in the protein. Separate calculations using the AMBER94 and OPLS2001 force fields were used to obtain vibrational frequencies for an energy minimized form of ubiquitin using the X-ray crystal structure (Vijay-Kumar, S.; Bugg, C. E.; Cook, W. J. Structure of Ubiquitin Refined at 1.8 A Resolution. J. Mol. Bio. 1987, 194, 531–544) as the starting geometry. These two different sets of vibrational frequencies resulted in remarkably similar values for internal energy at room temperature (17.2 and 17.5 eV, respectively), indicating that this calculation is not very dependent on the force field employed. Internal energies 6 and 12 eV higher (to account for the recombination energy of 1 and 2 electrons, respectively) than those determined at room temperature were compared with various thermal internal energies to estimate the effective temperature of the product ion after electron capture.
Price, W. D.; Williams, E. R. Activation of Peptide Ions by Blackbody Radiation: Factors that Lead to Dissociation Kinetics in the Rapid Energy Exchange Limit. J. Phys. Chem. A. 1997, 101, 8844–8852.
Dunbar, R. C. Infrared Radiative Cooling of Gas-Phase Ions. Mass Spectrom. Rev. 1992, 11, 309–339.
Bowen, R. D.; Maccoll, A. Low Energy, Low Temperature Mass Spectra 2—Low Energy, Low Temperature Mass Spectra of Some Small Saturated Alcohols and Ethers. Org. Mass Spectrom. 1984, 19, 379–384.
Holmes, J. L.; Yuan, D.; Rye, R. T. B. Metastable Ion Studies: VII—Loss of Water from the Molecular Ion of Cyclopentanol. Org. Mass Spectrom. 1977, 12, 254–257.
Sena, M.; Riveros, J. M. Thermal Dissociation of Acetophenone Molecular Ions Activated by Infrared Radiation. J. Phys. Chem. A. 1997, 101, 4384–4391.
Sena, M.; Riveros, J. M. Dissociation of p-Cymene Molecular Ions Induced by Thermal Radiation. Int. J. Mass Spectrom. 2003, 227, 135–145.
Wong, R. L.; Robinson, E. W.; Williams, E. R. Activation of Protonated Peptides and Molecular Ions of Small Molecules Using Heated Filaments in Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry. Int. J. Mass Spectrom. 2004, 234, 1–9.
Breuker, K.; Oh, H. B.; Lin, C.; Carpenter, B. K.; McLafferty, F. W. Nonergodic and Conformational Control of the Electron Capture Dissociation of Protein Cations. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 14011–14016.
Turecek, F. N-C-α Bond Dissociation Energies and Kinetics in Amide and Peptide Radicals: Is the Dissociation a Nonergodic Process?. J. Am. Chem. Soc. 2003, 125, 5954–5963.
Turecek, F.; Syrstad, E. A. Mechanism and Energetics of Intramolecular Hydrogen Transfer in Amide and Peptide Radicals and Cation Radicals. J. Am. Chem. Soc. 2003, 125, 3353–3369.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published online August 4, 2006
Rights and permissions
About this article
Cite this article
Robinson, E.W., Leib, R.D. & Williams, E.R. The role of conformation on electron capture dissociation of ubiquitin. The official journal of The American Society for Mass Spectrometry 17, 1470–1479 (2006). https://doi.org/10.1016/j.jasms.2006.06.027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jasms.2006.06.027