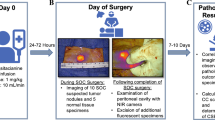
Abstract
Completeness of cytoreduction is an independent prognostic factor after cure-intended surgery for peritoneal carcinomatosis. NV1066, a genetically engineered herpes simplex virus carrying the transgene for green fluorescent protein, selectively infects cancer cells. We sought to determine the feasibility of virally directed fluorescent imaging in the intraoperative detection of minimal residual disease after cytoreductive surgery. NV1066 infected human gastric cancer cells, OCUM-2MD3, and mesothelioma JMN cells at all doses. The infected cells expressed green fluorescent protein and were killed. OCUM-2MD3, and mesothelioma JMN cells at all doses. Peritoneal carcinomatosis was established in mice by injection of OCUM cells into the peritoneal cavity. Forty-eight hours after intraperitoneal injection of NV1066, two experienced surgeons resected all visible disease and identified mice free of disease. Eight of 13 mice thought to be free of disease were found to have residual disease as identified by green fluorescence (mean number of observations: 5; range: 1–9). Residual disease was most frequently observed in the retroperitoneum, pelvis, peritoneal surface, and liver. Specificity of NV1066 infection to tumor nodules was confirmed by immunohistochemistry and by polymerase chain reaction for viral gene. Virally directed fluorescent imaging, a novel molecular imaging technology, can be used for real-time visualization of minimal residual disease after cytoreductive surgery and can improve the completeness of cure-intended resection.
Similar content being viewed by others
References
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21(20):3737–3743.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22(16):3284–3292.
Verwaal V, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder F. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005;12(1):65–71.
Bennett JJ, Delman KA, Burt BM, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther 2002;9(11):935–945.
Stanziale SF, Stiles BM, Bhargava A, Kerns SA, Kalakonda N, Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum Gene Ther 2004;15(6):609–618.
Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y. Oncolytic herpes virus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum Gene Ther 2002; 13(10):1213–1223.
Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 2. Perioperative intraperitoneal chemotherapy. Oncology (Huntington) 2004; 18(2):207–219.
Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 1. Patterns of dissemination and treatment options. Oncology (Huntington) 2004;18(1):51–59.
Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000; 88(2):358–363.
Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 2001; 27(3):239–243.
Yonemura Y, Bandou E, Kinoshita K, et al. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am 2003;12(3):635–648.
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240(5):808–816.
Wilson JJ, Jones H, Burock M, et al. Patterns of recurrence in patients treated with photodynamic therapy for intraperitoneal carcinomatosis and sarcomatosis. Int J Oncol 2004; 24(3):711–717.
Bennett JJ, Adusumilli P, Petrowsky H, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207). FASEB J 2004;18(9):1001–1003.
Bennett JJ, Kooby DA, Delman K, et al. Antitumor efficacy of regional oncolytic viral therapy for peritoneally disseminated cancer. J Mol Med 2000;78(3):166–174.
Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000;7(10):867–874.
Fong Y, Kemeny N, Jarnagin W, et al. Phase 1 study of a replication-competent herpes simplex oncolytic virus for treatment of hepatic colorectal metastases. Proc Am Soc Clin Oncol 2002;21:8a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by AstraZeneca Cancer Research and Prevention fellowship (P.S.A), training grant T 32 CA09501 (D.P.E and K.J.H.), grants RO1 CA 76416 and RO1 CA/DK80982 (Y.F.) from the National Institutes of Health, grant BC024118 from the U.S. Army (Y.F.), grant IMG0402501 from the Susan G. Komen Foundation (Y.F. and P.S.A), and grant 032047 from the Flight Attendant Medical Research Institute (Y.F. and P.S.A).
Rights and permissions
About this article
Cite this article
Adusumilli, P.S., Eisenberg, D.P., Chun, Y.S. et al. Virally directed fluorescent imaging improves diagnostic sensitivity in the detection of minimal residual disease after potentially curative cytoreductive surgery. J Gastrointest Surg 9, 1138–1147 (2005). https://doi.org/10.1016/j.gassur.2005.06.029
Issue Date:
DOI: https://doi.org/10.1016/j.gassur.2005.06.029