Abstract
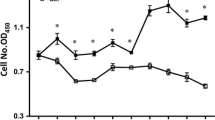
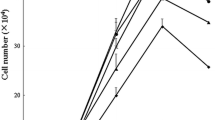
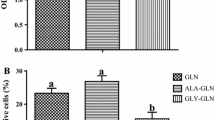
Glutamine is an essential nutrient for cell integrity during acidotic states such as shock, but the effect of extracellular pH on intestinal mucosal cell glutamine uptake is poorly understood. The purpose of this in vitro study was to investigate the intracellular signaling pathways involved in controlling intestinal glutamine transport during acidosis. Lowering the pH in the cell culture medium resulted in an increase in glutamine transport activity in a time- and pH-dependent fashion. Chronic acidosis (pH 6.6 for 48 hours) resulted in a twofold increase in glutamine transport activity (1.63 ± 0.25 nmole/mg protein/ minute in acidosis vs. 0.78 ± 0.11 nmole/mg protein/minute in control) and a threefold increase in glutamine transport gene ATB messenger RNA levels. This acidosis-induced increase in glutamine transport activity was due to a stimulation of transporter maximal transport capacity (Vmax 13.6 ± 0.73 nmole/mg protein/minute in acidosis vs. 6.3 ± 0.46 nmole/mg protein/minute in control) rather than a change in transporter affinity (Km = 0.23 ± 0.02 mmol/L glutamine in acidosis vs. 0.19 ± 0.02 mmol/ L glutamine in control). This acidosis-stimulated glutamine transport activity was blocked by actinomycin-D or cycloheximide. Cellular mitogen-activated protein kinase (MAPK) MEK1/2 and p42/44 levels were elevated in acidotic cells, and the acidosis-induced glutamine transport activity was blocked by the MAPK MEK 1 inhibitor PD 98059. Acidosis stimulates glutamine transport in Caco-2 cells via signaling pathways that lead to transcription of the glutamine transporter gene and translation of functional transporters. Mitogen-activated protein kinases are key intracellular regulators involved in this signal transduction cascade. An increased availability of glutamine to cells subjected to redox stress may help in maintaining cellular integrity.
Similar content being viewed by others
References
Souba WW. Glutamine: Physiology, Biochemistry and Nutrition in Critical Illness. Austin: Landes RG company, 1991.
Stevens BR. Amino acid transport in intestine. Im Kilberg MS, Haussinger D, eds. Mammalian Amino Acid Transport. New York: Plenum Press, 1992, 149–161.
Diamond JM, Karasov WH. Adaptive regulation of intestinal nutrient transporters. Proc Natl Acad Sci U S A 1987;84:2242–2245.
Levine GM. Growth of the Gastrointestinal Tract. In Moris-set J, Solomon TE, eds. Gastrointestinal Hormones and Growth Factors. Baton Raton: CRC Press, 1991, 245–253.
Relman AS. Metabolic consequences of acid-base disorders. Kidney Int 1972;1:347–459.
Mitchell JH, Wildenthal K, Johnson RL Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int 1972;1:375–389.
Brobst D. Pathophysiologic and adaptive changes in acidbase disorders. J Am Vet Med Assoc 1983;183:773–780.
Deferrari G, Garibotto G, Robaudo C, Saffioti S, Russo R, Sofia A Protein and amino acid metabolism in splanchnic organs in metabolic acidosis. Miner Electrolyte Metab 1997; 23:229–233.
Swenson ER. Metabolic acidosis. Respir Care 2001;46:342–353.
Gauthier PM, Szerlip HM. Metabolic acidosis in the intensive care unit. Crit Care Clin 2002;18:289–308.
Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 1995;15:133–159.
Welbourne TC, Joshi S. Interorgan glutamine metabolism during acidosis. JPEN J Parenter Enteral Nutr 1990; 14(4 Suppl):77S-85S.
Watford M, Chellaraj V, Ismat A, Brown P, Raman P. Hepatic glutamine metabolism. Nutrition 2002; 18:301–303.
Schrock H, Goldstein L. Interorgan relationships for glutamine metabolism in normal and acidotic rats. Am J Physiol 1981;240:E519-E525.
Tamarappoo BK, Joshi S, Welbourne TC. Interorgan glutamine flow regulation in metabolic acidosis. Miner. Electrolyte Metab 1990;16:322–330.
Foreman JW, Reynolds RA, Ginkinger K, Stanton S. Effect of acidosis on glutamine transport by isolated rat renal brushborder and basolateral-membrane vesicles. Biochem J 1983; 212:713–720.
Windus DW, Cohn DE, Klahr S, Hammerman MR. Glutamine transport in renal basolateral vesicles from dogs with metabolic acidosis. Am J Physiol 1984;246:F78-F86.
Souba WW, Pan M, Stevens BR. Kinetics of the sodiumdependent glutamine transporter in human intestinal cell confluent monolayers. Biochem Biophys Res Commun 1992;188:746–753.
Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989; 96:736–749.
Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst 1997;14:221–286.
Pan M, Stevens BR. Differentiation- and protein kinase C-dependent regulation of alanine transport via system B. J Biol Chem 1995;270:3582–3587.
Salloum RM, Stevens BR, Schultz GS, Souba WW. Regulation of small intestinal glutamine transport by epidermal growth factor. Surgery 1993;113:552–559.
O’Brian CA, Ward NE. Biology of the protein kinase C family. Cancer Metastasis Rev 1989;8:199–214.
Pan M, Stevens BR. Protein kinase C-dependent regulation of L-arginine transport activity in Caco-2 intestinal cells. Biochim Biophys Acta 1995; 1239:27–32.
Pan M, Wolfgang CA, Karinch AM, Lin C, Meng Q, Vary TC, Souba WW. Protein kinase C activation of intestinal glutamine transport is mediated by mitogen-activated protein kinases. J Surg Res 2002;106:137–144.
Pan M, Meng QH, Wolfgang CL, Lin CM, Karinch AM, VaryTC, Souba WW. Activation of intestinal arginine transport by protein kinase C is mediated by mitogen-activated protein kinases. J Gastrointest Surg 2002;6:876–882.
Tamaoki T, Nakano H. Potent and specific inhibitors of protein kinase C of microbial origin. Biotechnology 1990;8:732–735.
Herbert JM, Seban E, Maffrand JP. Characterization of specific binding sites for [3H]-staurosporine on various protein kinases. Biochem Biophys Res Commun 1990;171:189–195.
Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002;298:1911–1912.
Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 1995;267:682–685.
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrinol Rev 2001;22:153–183.
Tsuganezawa H, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int 2002;62:41–50.
Feifel E, Obexer P, Andratsch M, Euler S, Taylor L, Tang A, Wei Y, Schramek H, Curthoys NP, Gstraunthaler G. p38 MAPK mediates acid-induced transcription of PEPCK in LLC-PK(l)-FBPase(+) cells. Am J Physiol Renal Physiol 2002;283:F678-F688.
Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature 1991;350:158–160.
Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL, Lee JC. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem 1997;272:12116–12121.
Author information
Authors and Affiliations
Additional information
Supported in part by a career development grant from The Society for Surgery of Alimentary Tract and grant DK-62165 from the National Institute of Diabetes and Digestive and Kidney Disease.
Rights and permissions
About this article
Cite this article
Epler, M.J., Souba, W.W., Meng, Q. et al. Metabolic acidosis stimulates intestinal glutamine absorption. J Gastrointest Surg 7, 1045–1052 (2003). https://doi.org/10.1016/j.gassur.2003.09.005
Issue Date:
DOI: https://doi.org/10.1016/j.gassur.2003.09.005