Abstract
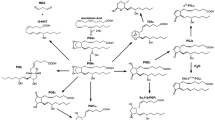
Prostaglandin H synthase (PGHS) is an autocatalytic enzyme which plays a key role in the arachidonic acid metabolic pathway. PGHS mediates the formation of prostaglandin H2, the precursor for a number of prostaglandins which are important in a wide variety of biological processes, including inflammation, blood clotting, renal function, and tumorigenesis. Here we present a Michaelis-Menten-style model for PGHS. A stability analysis determines when the reaction becomes self-sustaining, and can help explain the regulation of PGHS activity in vivo. We also consider a quasi-steady-state approximation (QSSA) for the model, and present conditions under which the QSSA is expected to be a good approximation. Applying the QSSA for this model can be useful in computationally intensive modeling endeavors involving PGHS.
Similar content being viewed by others
References
Bambai, B., Kulmacz, R.J., 2000. Prostaglandin H synthase. J. Biol. Chem. 275, 27608–27614.
Baron, J.A., Sandler, R.S., 2000. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu. Rev. Med. 51, 511–523.
Borghans, J.A.M., De Boer, R.J., Segel, L.A., 1996. Extending the quasi-steady state approximation by changing variables. B. Math. Biol. 58, 43–63.
Char, B.W., Russo, M.F., 1994. Automatic identification of time scales in enzyme kinetics models. In: Proceedings of the International Symposium on Symbolic and Algebraic Computation, Oxford, UK, July 20–22. Association of Computing Machinery.
Dietz, R., Nastainczyk, W., Ruf, H.H., 1988. Higher oxidation-states of prostaglandin-H synthase—rapid electronic spectroscopy detected 2 spectral intermediates during the peroxidase reaction with prostaglandin-G2. Eur. J. Biochem. 171, 321–328.
Edelstein-Keshet, L., 1988. Mathematical Models in Biology. Random House, New York.
Fenichel, N., 1971. Persistence and smoothness of invariant manifolds for flows. Indiana U. Math. J. 21, 193–226.
Fersht, A., 1985. Enzyme Structure and Mechanism. W.H. Freeman and Company, New York.
Ford-Hutchinson, A.W., Gresser, M., Young, R.N., 1994. 5-lipoxygenase. Annu. Rev. Biochem. 63, 383–417.
Funk, C.D, 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875.
Giovannucci, E., 1999. The prevention of colorectal cancer by aspirin use. Biomed. Pharmacother. 53, 303–308.
Hawkey, C.J., 2002. Cyclooxygenase inhibition: between the devil and the deep blue sea. Gut 50(Suppl. III), iii25–iii30.
Hazelton, W.D., Tien, J.H., Donato, V.W., Sparks, R., Ulrich, C.M., 2004. Prostaglandin H synthases: members of a class of quasi-linear threshold switches. Biochem. Pharmacol. 68, 423–432.
Jones, C.K.R.T., 1994. Geometric singular perturbation theory. In: Arnold, L. (Ed.), Dynamical Systems, Montecatini Terme. Lecture Notes in Mathematics, vol. 1609. Springer-Verlag, Berlin, pp. 44–118.
Kulmacz, R.J., 1998. Cellular regulation of prostaglandin H synthase catalysis. FEBS Lett. 430, 154–157.
Lin, C.C., Segel, L.A., 1974. Mathematics Applied to Deterministic Problems in the Natural Sciences. Macmillan Publishing Company, Inc, New York.
Lipsky, P.E., Brooks, P., Crofford, L.J., DuBois, R., Graham, D., Simon, L.S., van de Putte, L.B., Abramson, S.B., 2001. Unresolved issues in the role of cyclooxygenase-2 in normal physiologic processes and disease. Arch. Intern. Med. 169, 913–920.
Lu, G., Tsai, A.L., van Wart, H.E., Kulmacz, R.J., 1999. Comparison of the peroxidase reaction kinetics of prostaglandin H synthase-1 and 2. J. Biol. Chem. 274, 16162–16167.
Mizuno, K., Yamamoto, S., Lands, W.E.M., 1982. Effects of non-steroidal anti-inflammatory drugs on fatty-acid cyclooxygenase and prostaglandin hydroperoxidase activities. Prostaglandins 23, 743–757.
Sandler, R.S. et al., 2003. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New Engl. J. Med. 348, 883–890.
Schnell, S., Maini, P.K., 2000. Enzyme kinetics at high enzyme concentration. B. Math. Biol. 62, 483–499.
Segel, L.A., 1988. On the validity of the steady state assumption of enzyme kinetics. B. Math. Biol. 50, 579–593.
Segel, L.A., Slemrod, M., 1989. The quasi-steady-state assumption: a case study in perturbation. SIAM Rev. 31, 446–477.
Smith, W.L., DeWitt, D.L., Garavito, R.M., 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69, 145–182.
Smith, W.L., Garavito, R.M., DeWitt, D.L., 1996. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and-2. J. Biol. Chem. 271, 33157–33160.
Smith, C.J. et al., 1998. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 95, 13313–13318.
Taketo, M.M., 1998. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II). J. Natl. Cancer I 90, 1609–1620.
Thun, M.J., Henley, S.J., Patrono, C., 2002. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer I 94, 252–266.
Turini, M.E., DuBois, R.N., 2002. Cyclooxygenase-2: a therapeutic target. Annu. Rev. Med. 53, 35–57.
Tzafriri, A.R., 2003. Michaelis-Menten kinetics at high enzyme concentrations. B. Math. Biol. 65, 1111–1129.
Vane, J.R., Bakhle, Y.S., Botting, R.M., 1998. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. 38, 97–120.
Wei, C., Kulmacz, R.J., Tsai, A.L., 1995. Comparison of branched-chain and tightly coupled reaction mechanisms for prostaglandin H synthase. Biochemistry 34, 8499–8512.
Williams, C.S., Mann, M., DuBois, R.N., 1999. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18, 7908–7916.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tien, J.H., Hazelton, W.D., Sparks, R. et al. A Michaelis-Menten-style model for the autocatalytic enzyme prostaglandin H synthase. Bull. Math. Biol. 67, 683–700 (2005). https://doi.org/10.1016/j.bulm.2004.09.007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/j.bulm.2004.09.007