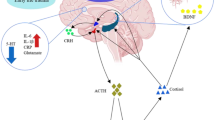
Abstract
Clinical studies have indicated a frequent coexistence of depression and diabetes. Both of these diseases are associated with similar changes in the structure and function of the central nervous system cells and with similar disturbances of cognitive processes. Some morphological and functional changes occurring in these diseases seem to result from exaggerated glucocorticoid, proinflammatory cytokine or glutamate action. Glucocorticoids induced by stress are known not only to affect synaptic plasticity but also to disturb brain glucose metabolism and decrease insulin sensitivity. Functional neuroimaging studies demonstrated altered glucose metabolism in the brains of depressed patients. Changes in the amount or activity of key metabolic enzymes and a lower sensitivity of insulin receptors have been detected in the brains of animal models of both of these diseases. Hence, excess glucocorticoids can lead to impaired insulin action and glucose metabolism, to limited energy supply for proper neuronal function and, consequently, to disturbed synaptic plasticity.
Similar content being viewed by others
Abbreviations
- AIR:
-
acute insulin response
- BBB:
-
bloodbrain barrier
- BDNF:
-
brain-derived neurotrophic factor
- CNS:
-
central nervous system
- CRF:
-
corticotropin-releasing factor
- CUMS:
-
chronic unpredictable mild stress
- GLP:
-
glucagonlike peptide
- GLUT:
-
glucose transporter
- GR:
-
glucocorticoid receptors
- HFD:
-
high-fat diet
- HK:
-
hexokinase
- HPA:
-
hypothalamus-pituitary-adrenal
- IGF:
-
insulin-like growth factor
- IRs:
-
insulin receptors
- IRS:
-
insulin receptor substrate
- MR:
-
mineralocorticoid receptors
- NGF:
-
nerve growth factor
- PVN:
-
hypothalamic paraventricular nucleus
- SOCS:
-
suppressor of cytokine signaling
- VMH:
-
hypothalamic ventromedial nucleus
References
Baldwin D, Apel J: Management of hyperglycemia in hospitalized patients with renal insufficiency or steroid-induced diabetes. Curr Diab Rep, 2013, 13, 114–120.
Banks WA, Owen JB, Erickson MA: Insulin in the brain: there and back again. Pharmacol Ther, 2012, 136, 82–93.
Beauquis J, Roig P, De Nicola AF, Saravia F: Neuronal plasticity and antidepressants in the diabetic brain. Ann N Y Acad Sci, 2009, 1153, 203–208.
Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R et al.: Role of brain insulin receptor in control of body weight and reproduction. Science, 2000, 289, 2122–2125.
Chiu SL, Chen CM, Cline HT: Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron, 2008, 58, 708–719.
Darsalia V, Mansouri S, Ortsäter H, Olverling A, No-zadze N, Kappe C, Iverfeldt K et al.: Glucagon-like peptide-I receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond), 2012, 122, 473–483.
Deuschle M: Effects of antidepressants on glucose metabolism and diabetes mellitus type 2 in adults. Curr Opin Psychiatry, 2013, 26, 60–65.
Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME: Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharm Biochem Behav, 2002, 71, 431–447.
Drevets WC, Price JL, Furey ML: Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct, 2008, 213, 93–118.
García-Díaz DF, Campion J, Milagro FI, Lomba A, Marzo F, Martínez JA: Chronic mild stress induces variations in locomotive behavior and metabolic rates in high fat fed rats. J Physiol Biochem, 2007, 63, 337–346.
Gejl M, Lerche S, Egefjord L, Brock B, Møller N, Vang K, Rodell AB et al.: Glucagon-like peptide-1 (GLP-1) raises blood-brain glucose transfer capacity and hexokinase activity in human brain. Front Neuroenergetics, 2013, 5, 2.
Gil-Lozano M, Pérez-Tilve D, Alvarez-Crespo M, Martís A, Fernandez AM, Catalina PAF, González-Matías L, Mallo F: GLP-1(7–36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology, 2010, 151, 2629–2640.
Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP: Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res, 2011, 222, 230–235.
Haj-ali V, Mohaddes G, Babri SH: Intracerebroventricular insulin improves spatial learning and memory in male Wistarrats. Behav Neurosci, 2009, 123, 1309–1314.
Hosokawa T, Momose T, Kasai K: Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic state. Prog Neuropsychopharmacol Biol Psychiatry, 2009, 33, 243–250.
Hoyer S, Lannert H: Long-term effects of corticosterone on behavior, oxidative and energy metabolism of parietotemporal cerebral cortex and hippocampus of rats: comparison to intracerebroventricular streptozotocin. J Neural Transm, 2008, 115, 1241–1249.
Hu H, Su L, Xu YQ, Zhang H, Wang LW: Behavioral and [F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression. Neuroscience, 2010, 169, 171–181.
Hung Y, Hsieh C, Chen Y, Pei D, Kuo S, Shen D, Sheu WH, Chen Y: Insulin sensitivity, proinflammatory markers and adiponectin in young males with different subtypes of depressive disorder. Clin Endocrinol, 2007, 67, 784–789.
Kanemaru K, Diksic M: The Flinders Sensitive Line of rats, a rat model of depression, has elevated brain glucose utilization when compared to normal rats and the Flinders Resistant Line of rats. Neurochem Int, 2009, 55, 655–661.
Khanam R, Najfi H, Akhtar M, Vohora D: Evaluation of venlafaxine on glucose homeostasis and oxidative stress in diabetic mice. Hum Exp Toxicol, 2012, 31, 1244–1250.
Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C et al.: Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab, 2007, 5, 438–449.
Lemaire V, Koehl M, Le Moal M, Abrous DN: Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA, 2000, 97, 11032–11037.
Li L, Li X, Zhou W, Messina JL: Acute psychological stress results in the rapid development of insulin resistance. J Endocrinol, 2013, 217, 175–184.
Magariños AM, McEwen BS: Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci USA, 2000, 97, 11056–11061.
Marano CM, Workman CI, Kramer E, Hermann CR, Ma Y, Dhawan V, Chaly T et al.: Longitual studies of cerebral glucose metabolism in late-life depression and normal aging. Int J Geriatr Psychiatry, 2013, 28, 417–423.
Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA: Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci, 2009, 29, 6734–6751.
McEwen BS, Magariños AM, Reagan LP: Studies of hormone action in the hippocampal formation: possible relevance to depression and diabetes. J Psychosom Res, 2002, 53, 883–890.
Mielke JG, Wang YT: Insulin, synaptic function, and opportunities for neuroprotection. Prog Mol Biol Transl Sci, 2011, 98, 133–186.
Nyirenda MJ, Welberg LAM, Seckl JR: Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol, 2001, 170, 653–660.
Pan Y, Hong Y, Zhang QY, Kong LD: Impaired hypotha-lamic insulin signaling in CUMS rats: restored by icariin and fluoxetine through inhibiting CRF system. Psychoneuroendocrinology, 2013, 38, 122–134.
Pandini G, Pace V, Copani A, Squatrito S, Milardi D, Vigneri R: Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology, 2013, 154, 375–387.
Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH: A novel neurotrophic property of glucagons-like+ peptide-1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther, 2002, 300, 958–966.
Pham K, Nacher J, Hof PR, McEwen BS: Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci, 2003, 17, 879–886.
Plaschke K, Müller D, Hoyer S: Effect of adrenalectomy and corticosterone substitution on glucose and glycogen metabolism in rat brain. J Neural Transm, 1996, 103, 89–100.
Porte D Jr, Baskin DG, Schwartz MW: Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes, 2005, 54, 1264–1276.
Porter DW, Kerr BD, Flatt PR, Holscher C, Gault VA: Four weeks administration of liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab, 2010, 12, 891–899.
Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pong-chaidecha A, Chattipakorn N, Chattipakorn SC: Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci, 2011, 88, 619–627.
Rasgon NL, Kenna HA: Insulin resistance in depressive disorders and Alzheimer’s disease: revisiting the missing link hypothesis. Neurobiol Aging, 2005, 26, Suppl 1, 103–107.
Regenold WT, Pratt M, Nekkalapu S, Shapiro PS, Kristian T, Fiskum G: Mitochondrial detachment in mood and psychotic disorders: Implications for brain energy metabolism and neurotrophic signalling. J Psych Res, 2012, 46, 95–104.
Saravia FE, Beauquis J, Revsin Y, Homo-Delarche F, de Kloet ER, De Nicola AF: Hippocampal neuropathology of diabetes mellitus is relieved by estrogen treatment. Cell Mol Neurobiol, 2006, 26, 943–957.
Scherer T, Lehnert H, Hallschmid M: Brain insulin and leptin signaling in metabolic control: from animal research to clinical application. Endocrinol Metab Clin North Am, 2013, 42, 109–125.
Skelin I, Sato H, Diksic M: Olfactory bulbectomy reduces cerebral glucose utilization: 2-[14C]deoxyglucose autoradiographic study. Brain Res Bull, 2008, 76, 485–492.
Tagliari B, Noschang CG, Ferreira AG, Ferrari OA, Feksa LR, Wannmacher CM, Dalmaz C, Wyse AT: Chronic variable stress impairs energy metabolism in prefrontal cortex and hippocampus of rats: prevention by chronic antioxidant treatment. Metab Brain Dis, 2010, 25, 169–176.
Vogt MC, Brüning JC: CNS insulin signaling in the control of energy homeostasis and glucose metabolism – from embryo to old age. Trends Endocrinol Metab, 2013, 24, 76–84.
Woods SC, Seeley RJ, Baskin DG, Schwartz MW: Insulin and the blood-brain barrier. Curr Pharm Des, 2003, 9, 795–800.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Detka, J., Kurek, A., Basta-Kaim, A. et al. Neuroendocrine link between stress, depression and diabetes. Pharmacol. Rep 65, 1591–1600 (2013). https://doi.org/10.1016/S1734-1140(13)71520-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1734-1140(13)71520-2