Abstract
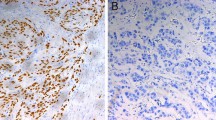
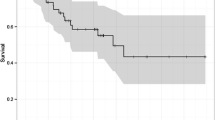
Altered expression of the genes that control apoptosis and proliferation may influence the response of cancer cells to cytotoxic agents. The primary aim of this study was to determine the role of the novel an-tiapoptotic and cell cycle gene, survivin, in apoptotsis and proliferation in esophageal cancer and to evaluate whether the survivin, p53, and bcl-2 status were able to predict a patient’s response to neoadjuvant therapy. A total of 104 patients with esophageal tumors were studied. Tumor tissue was immunostained for survivin, p53, and bcl-2 proteins. Proliferative and apoptotic activity was measured using ki-67 immu-nohistochemical analysis and the TUNEL method, respectively. Forty-eight patients whose pretreat-ment biopsies were analyzed received neoadjuvant chemoradiation therapy or chemotherapy followed by surgery. Outcome was graded as a complete response, a partial response, or no response according to the results of histologic examination and CT imaging. Expression of survivin was found to correlate significantly with the proliferative index but not the apoptotic index. Patients who received neoadjuvant treatment were more likely to achieve a complete response if their tumors had high proliferative activity, and p53 positive tumors were more likely to contain residual tumor after treatment. In conclusion, survivin expression appears to foster proliferative activity in esophageal cancer, and tumors with a high proliferative index or a functioning p53 gene are more responsive to neoadjuvant chemoradiation therapy.
Similar content being viewed by others
References
Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunder-son L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W, Minsky BD, Roth JA. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979–1984.
Medical Research Council (MRC) Upper GI Tract Cancer Group. Medical Research Council (MRC) randomised phase III trial of surgery with or without pre-operative chemotherapy in resectable cancer of the oesophagus [abstr]. Br J Cancer 2000;83(Suppl 1):1.
Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TPJ. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462–467.
Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1996;337:161–167.
Kerr JF, Winterford CM, Harmon BV. Apoptosis: Its significance in cancer and cancer therapy. Cancer 1994;73:2013–2226.
Lane DP. P53, guardian of the genome. Nature 1992;358:15–16.
McConkey DJ, Chandra J, Wright S, Plunkett W, McDonnell TJ, Reed JC, Keating M. Apoptosis sensitivity in chronic lymphocytic leukaemia is determined by endogenous endonuclease content and relative expression of BCL-2 and BAX. J Immunol 1996;156:2624–2630.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997;3:917–921.
Deveraux QL, Reed JC. IAP family proteins—Suppressors of apoptosis. Genes Dev 1999;13:239–252.
Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting.JBiol Chem 1998;273:11177–11182.
Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998;396:580–584.
Kawasaki H, Altieri DC, Lu C-D, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 1998; 58:5071–5074.
Sarela AI, Macadam RC, Farmery SM, Markham AF, Guil-lou PJ. Expression of the anti-apoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut 2000;46:645–650.
Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet 1999;351:882–883.
Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the anti-apoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med 1999;341:452–453.
Olie RA, Simoes-Wust AP, Baumann B, Leech SH, Fabbro D, Stahel RA, Zangemeister-Wittke U. A novel anti-sense oligonucleotide targeting survivin expression induces apoptosis and sensitises lung cancer cells to chemotherapy. Cancer Res 2000;60:2805–2809.
Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein in pancreatic ad-enocarcinoma. Br J Cancer 2002;86:886–892.
Kawasaki H, Toyodo M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation and angiogenesis during human colorectal tumorigenesis. Cancer 2001;91:2026–2032.
Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, Nakano T, Suzuki A. Survivin promotes cell proliferation in human hepa-tocellular carcinoma. Hepatology 2000;31:1080–1085.
Gianini R, Jarboe E, Orlicky D, Frost M, Bobak J, Lehner R, Shroyer KR. Expression of survivin in normal, hyperplastic and neoplastic colonic mucosa. Hum Pathol 2001;32:119–125.
Fekuda S, Pelus LM. Regulation of the inhibitor-of-apop-tosis family member survivin in normal cord blood and bone marrow CD34+ cells by haematopoietic growth factors: Implication of survivin expression in normal haemato-poiesis. Blood 2001;98:2091–2100.
Lu C, Altieri D, Tanigawa N. Expression of a novel antiap-optosis gene, survivin, correlated with tumour cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 1998;58:1808–1812.
Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134.
Katada N, Hinder R, Smyrk TC, Hirabayashi N, Perdikis G, Lund RJ, Woodward T, Klinger PJ. Apoptosis is inhibited early in the dysplasia-carcinoma sequence of Barrett’s esophagus. Arch Surg 1997;132:728–733.
Kitamura K, Sacki H, Kawaguchi H, Araki K, Ohno S, Ku-wano H, Maehara Y, Sugimachi K. Immunohistochemical status of the p53 and ki-67 antigen using biopsied specimens can predict a sensitivity to neoadjuvant therapy in patients with esophageal cancer. Hepatogastroenterolgy 2000; 47:419–423.
Miyata H, Yuichiro D, Shiozaki H, Inoue M, Yano M, Fuji-wara Y, Yamamoto H, Nishioka K, Kishi K, Monden M. CDC25B and p53 are independently implicated in radiation sensitivity for human esophageal cancer. Clin Cancer Res 2000;6:4859–4865.
Author information
Authors and Affiliations
Additional information
Supported by grants from the Royal College of Surgeons of England and Yorkshire Cancer Research.
An erratum to this article is available at http://dx.doi.org/10.1016/S1091-255X(03)00068-4.
Rights and permissions
About this article
Cite this article
Beardsmore, D.M., Verbeke, C.S., Davies, C.L. et al. Apoptotic and proliferative indexes in esophageal cancer: Predictors of response to neoadjuvant therapy apoptosis and proliferation in esophageal cancer. J Gastrointest Surg 7, 77–87 (2003). https://doi.org/10.1016/S1091-255X(02)00141-5
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1091-255X(02)00141-5