Abstract
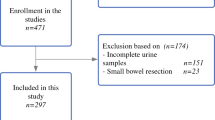
Intestinal barrier failure and subsequent bacterial translocation have been implicated in the development of organ dysfunction and septic complications associated with severe acute pancreatitis. Splanchnic hypoperfusion and ischemia/reperfusion injury have been postulated as a cause of increased intestinal permeability. The urinary concentration of intestinal fatty acid binding protein (IFABP) has been shown to be a sensitive marker of intestinal ischemia, with increased levels being associated with ischemia/reperfusion. The aim of the current study was to assess the relationship between excretion of IFABP in urine, gut mucosal barrier failure (intestinal hyperpermeability and systemic exposure to endotoxemia), and clinical severity. Patients with a clinical and biochemical diagnosis of acute pancreatitis were studied within 72 hours of onset of pain. Polyethylene glycol probes of 3350 kDa and 400 kDa were administered enterally, and the ratio of the percentage of retrieval of each probe after renal excretion was used as a measure of intestinal macromolecular permeability. Collected urine was also used to determine the IFABP concentration (IFABP-c) and total IFABP (IFABP-t) excreted over the 24-hour period, using an enzyme-linked immun-osorbent assay technique. The systemic inflammatory response was estimated from peak 0 to 72-hour plasma C-reactive protein levels, and systemic exposure to endotoxins was measured using serum IgM en-dotoxin cytoplasmic antibody (EndoCAb) levels. The severity of the attack was assessed on the basis of the Atlanta criteria. Sixty-one patients with acute pancreatitis (severe in 19) and 12 healthy control subjects were studied. Compared to mild attacks, severe attacks were associated with significantly higher urinary IFABP-c (median 1092 pg/ml vs. 84 pg/ml; P < 0.001) and IFABP-t (median 1.14 μg vs. 0.21 |μg; P = 0.003). Furthermore, the control group had significantly lower IFABP-c (median 37 pg/ml; P = 0.029) and IFABP-t (median 0.06 μg; P = 0.005) than patients with mild attacks. IFABP correlated positively with the polyethylene glycol 3350 percentage retrieval (r = 0.50; P < 0.001), CRP (r = 0.51; P < 0.001), and inversely with serum IgM EndoCAb levels (r = —0.32; P = 0.02). The results of this study support the hypothesis that splanchnic hypoperfusion contributes to the loss of intestinal mucosal integrity associated with a severe attack of pancreatitis.
Similar content being viewed by others
References
Bradley EL III, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg 1991; 161:19–24.
Forsmark CE, Toskes PP. Acute pancreatitis. Medical management. Crit Care Clin 1995;11:295–309.
Larvin M, McMahon MJ. Apache-II score for assessment and monitoring of acute pancreatitis. Lancet 1989;22:201–205.
Mann DV, Hershman MJ, Hittinger R, Glazer G. Multi-centre audit of death in acute pancreatitis. Br J Surg 1994; 81:890–893.
Renner IG, Savage WT III, Pantoja JL, Renner VJ. Death due to acute pancreatitis: A prospective analysis of 405 autopsy cases. Dig Dis Sci 1985;30:1005–1018.
Buggy BP, Nostrant TT. Lethal pancreatitis. Am J Gastro-enterol 1983;78:810–814.
Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis—A prospective clinical study. Gastroenterology 1986;91:433–438.
Gerzof SA, Banks PA, Robbins AH, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology 1987;93:1315–1320.
Bassi C, Falconi M, Girelli R, et al. Microbiological findings in severe pancreatitis. Surg Res Commun 1989;5:1–4.
Bradley ELIII. A clinically based classification system for acute pancreatitis. Arch Surg 1993;128:586–590.
Büchler M, Malfertheiner P, Friess H, et al. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology 1992;103:1902–1908.
Fedorak IJ, Ko TC, Djuricin G, et al. Secondary pancreatic infections: Are they distinct clinical entities?. Surgery 1992; 112:824;-831.
Malangoni MA, Shallcross JC, Seiler JG, et al. Factors contributing to fatal outcome after treatment of pancreatic abscess. Ann Surg 1986;203:605–613.
Bassi C, Vesentini S, Nifosi F, et al. Pancreatic abscess and other pus-harboring collections related to pancreatitis: A review of 108 cases. World J Surg 1990;14:505–512.
Lumsden A, Bradley EL III. Secondary pancreatic infection. Surg Gynecol Obstet 1990;170:459–467.
Swank GM, Deitch EA. Role of the gut in multiple organ failure: Bacterial translocation and permeability changes. World J Surg 1996;20:411–417.
Weinstein L, Swartz MN. Pathogenic properties of invading micro-organisms. In Sodeman WA Jr, Sodeman WA, eds. Pathologic Physiology: Mechanisms of Disease. Philadelphia: Saunders, 1974, pp 466–468.
Connor J, Fine J, Kusano K, et al. Potentiation by endot-oxin of responses associated with increases in calcium conductance. Proc Natl Acad Sci USA 1973;70:3301–3304.
Foulis AK, Murray WR, Galloway D, et al. Endotoxemia and complement activation in acute pancreatitis in man. Gut 1982;23:656–661.
Exley AR, Leese T, Holliday MP, et al. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut 1992;33:1126–1128.
Liehr H, Grun M, Seeling R, Seeling H-P. Endotoxi-namie bei akuter Pankreatitis. Leber Magen Darm 1980; 10:259–264.
Wang XD, Wang Q, Andersson R, Ihse I. Alterations in intestinal function in acute pancreatitis in an experimental model. Br J Surg 1996;83:1537–1543.
Ryan CM, Schmidt J, Lewandrowski K, et al. Gut macro-molecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology 1993;104:890–895.
Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: Correlation with endotoxemia, organ failure, and mortality. JGastrointest Surg 1999;3:252–262.
Runkel NS, Moody FG, Smith GS, et al. The role of the gut in the development of sepsis in acute pancreatitis. J Surg Res 1991;51:18–23.
Gianotti L, Munda R, Alexander JW. Pancreatitis-induced microbial translocation: A study of the mechanisms. Res Surg 1992;4:87–91.
Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 1992;216:117–134.
Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The ‘undrained abscess’ of multiple organ failure. Ann Surg 1993;218:111–119.
Meakins JL, Marshall JC. The gastrointestinal tract: The "motor" of multiple organ failure. Arch Surg 1986; 121:197.
Border JR, Hassett J, LaDuca J, et al. Gut origin septic states in blunt trauma (ISS = 40) in the ICU. Ann Surg 1987;206:427–448.
Kivilaakso E, Valtonen VV, Malkamaki M, et al. Endotoxaemia and acute pancreatitis: Correlation between the severity of the disease and the anti-enterobacterial common antigen antibody titre. Gut 1984;25:1065–1070.
Windsor JA, Fearon KCH, Ross JA, et al. The role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg 1993;80:1042–1046.
Tarpila E, Nystrom P-O, Franzen L, Ihse I. Bacterial translocation during acute pancreatitis in rats. Eur J Surg 1993; 159:109–113.
Warshaw AL. Inflammatory masses following acute pancreatitis. Surg Clin North Am 1974;54:620–637.
Webster MW, Pasculle AW, Myerowitz RL, et al. Postin-duction bacteremia in experimental acute pancreatitis. Am J Surg 1979;138:418–420.
Byrne JJ, Joison J. Bacterial regurgitation in experimental pancreatitis. Am J Surg 1964;107:317–320.
Keynes WM. A nonpancreatic source of the proteolytic enzyme amidase and bacteriology in experimental acute pancreatitis. Ann Surg 1980;191:187–199.
Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg 1986;121:196–208.
Morris SE, Navaratnam N, Townsend CM, Herndon DN. Decreased mesenteric blood flow independently promotes bacterial translocation in chronically instrumented sheep. Surg Forum 1989;40:88–90.
Navaratnam NS, Morris C, Townsend C, et al. Bacterial translocation and selective mesenteric artery perfusion with nitroprusside in an ovine model [abstr]. Int J Radiat Oncol Biol Phys 1989;21:240.
Tokyay R, Zeigler ST, Traber DL, et al. Postburn gastrointestinal vasoconstriction increases bacterial and endo-toxin translocation. J Appl Physiol 1993;74:1521–1527.
Redan JA, Rush BF, Lysz TW, et al. Organ distribution of gut-derived bacteria caused by bowel manipulation ischemia. Am J Surg 1990;159:85–89.
Sori AJ, Rush BF, Lysz TW, Smith S, Machiedo GW. The gut as a source of sepsis after hemorrhagic shock. Am J Surg 1988;155:187–192.
Baker JW, Deitch EA, Berg RD, Specian RD. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma 1988;28:896–906.
Horton JW, Walker PB. Oxygen radicals, lipid peroxida-tion, and permeability changes after intestinal ischemia and reperfusion. J Appl Physiol 1993;74:1515–1520.
Kubes P. Ischemia-reperfusion in feline small intestine: A role fornitric oxide. Am J Physiol 1993;264:G143-G149.
Deitch EA, Berg R. Bacterial translocation from the gut: A mechanism of infection. J Burn Care Rehabil 1987;8:475–482.
Tancrede CH, Andremont AO. Bacterial translocation, and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis 1985;152:99–103.
Berg RD. Bacterial translocation from the gastrointestinal tracts of mice receiving immunosuppressive chemothera-peutic agents. Curr Microbiol 1983;8:285–292.
Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun 1982;27:461–467.
Maddaus MA, Wells CL, Platt JL, et al. Effect of T cell modulation on the translocation of the bacteria from the gut and mesenteric lymph nodes. Ann Surg 1988;207:387–398.
Deitch EA, Maejima K, Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma 1985;25:385–392.
Steffen EK, Berg RD. Relationship between cecal population levels and indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun 1983;39:1252–1259.
Berg RD, Owen WE. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect Immun 1979;25:820–827.
Berg RD. Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobi-otic or antibiotic-decontaminated mice. Infect Immun 1980;29:1073–1081.
Andersson R, Wang XD, Ihse I. The influence of abdominal sepsis on acute pancreatitis in rats: A study on mortality, permeability, arterial pressure, and intestinal blood flow. Pancreas 1995;11:365–373.
Ockner RK, Manning JA. Fatty acid-binding protein in small intestine. J Clin Invest 1974;54:326–338.
Robinson JW, Mirkovitch V. The recovery of function and microcirculation in small intestinal loops following ischemia. Gut 1972;13:784–789.
Gollin G, Marks C, Marks WH. Intestinal fatty acid binding protein in serum and urine reflects early ischemic injury to the small bowel. Surgery 1993;113:545–551.
Kanda T, Nakatomi Y, Ishikawa H, et al. Intestinal fatty-acid binding protein as a sensitive marker of intestinal is-chaemia. Dig Dis Sci 1992;37:1362–1367.
Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: A physiologically based classification system. Crit Care Med 1981;9:591–597.
Ryan CM, Yarmush ML, Tompkins RG. Separation and quantification of polyethylene glycols 400 and 3350 from human urine by high-performance liquid chromatography. J Pharm Sci 1992;81:350–352.
Barclay GR, Scott BB. Serological relationships between Escherichia coli and Salmonella smooth- and rough-mutant lipopolysaccharides as revealed by enzyme-linked immun-osorbent assay for human immunoglobulin G antiendotoxin antibodies. Infect Immun 1987;55:2706–2714.
Barclay GR, Scott BB, Wright IH, et al. Changes in antiendotoxin IgG antibody and endotoxaemia in three cases of gram-negative septic shock. Circ Shock 1989;29:93–106.
Kanda T, Fujii H, Tani T, et al. Intestinal fatty-acid binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996;110:339–343.
Lieberman JM, Marks WH, Cohn S, et al. Organ failure, infection, and the systemic inflammatory response syndrome are associated with elevated levels of urinary intestinal fatty acid binding protein: Study of 100 consecutive patients in a surgical intensive care unit. J Trauma Infect Crit Care 1998;45:900–905.
Roitt I, Brostoff J, Male D. Immunology, 3rd ed. London: Mosby-Year Book Europe Ltd, 1993.
Granger DN, McCord D, Parks DA, Halloworth MB. Xan-thine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology 1986;90:80–84.
Parks DA, Williams TK, Beckman JS. Conversion of xan-thine dehydrogenase to oxidase in ischemic rat intestine: A re-evaluation. Am J Physiol 1988;54;G768-G774.
Payne D, Kubes P. Nitric oxide donors reduce the rise in reperfusion-induced intestinal mucosal permeability. Am J Physiol 1993;265:G189-G195.
Cipolle MD, Pasqualle MD, Ferra FB. Secondary organ dysfunction: From clinical perspectives to molecular mediators. Circ Shock 1993;9:261–298.
Hotz HG, Foitzik T, Schulzke JD, et al. Reduced capillary blood flow is not related to increased ion permeability of the colon in acute pancreatitis [in German]. Langenbecks Arch Chir Suppl Kongressbd 1997;114:413–415.
Klar E, Mall G, Messmer K, et al. Improvement of impaired pancreatic microcirculation by isovolemic hemodilution protects pancreatic morphology in acute biliary pancreatitis. Surg Gynecol Obstet 1993;176:144–150.
Hunt DR, Mildenhall P. Etiology of stricture of the colon associated with pancreatitis. Am J Dig Dis 1975;20:941–946.
Russell JC, Welch JP, Clark DG, et al. Colonic complications of acute pancreatitis and pancreatic abscess. Am J Surg 1983;146:558–564.
Jensen K, Bradley EL III. Mesenteric venous infarction in acute pancreatitis. Int J Pancreatol 1989;5:213–219.
Buttenschoen K, Berger D, Hiki N, et al. Endotoxin and antiendotoxin antibodies in patients with acute pancreatitis. Eur J Surgery 2000;166:459–466.
Michie HR, Manogue KR, Spriggs D, et al. Detection of circulating tumor necrosis factor during endotoxemia in humans. N Engl J Med 1988;318:1481–1486.
O’Dwyer ST, Michie HR, Ziegler TR, et al. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg 1988;123:1459–1464.
Walker RI, Porvaznilk MJ. Disruption of the permeability barrier (zona occludens) between intestinal epithelial cells by lethal doses of endotoxin. Infect Immun 1978;21:655–658.
Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol 1979;28:293–350.
Deitch EA, Berg R, Specian R. Endotoxin promotes the translo-cation of bacteria from the gut. Arch Surg 1987;122:185–190.
Curley PJ, McMahon MJ, Lancaster F, et al. Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: Relationship to endotoxin, interleukin 6 and disease severity. Br J Surg 1993;80:1312–1315.
Garcia-Sabrido JL, Valdecantos E, Bastida E, Tellado JM. The anergic state as a predictor of pancreatic sepsis. Zen-trabl Chir 1989;114:114–120.
Curley P, Nestor M, Collins K, et al. Decreased interleu-kin-2 production in murine acute pancreatitis: Potential for immunomodulation. Gastroenterology 1996;110:583–588.
Widdison AL, Karanjia ND, Alvarez C, Reber HA. Reticu-loendothelial function, and efficacy of levamisol for the treatment of pancreatic infection in acute necrotizing pancreatitis. Am J Surg 1992;163:100–104.
Summer RW, Kent TH. Effects of altered propulsion on rat small intestinal flora. Gastroenterology 1970;59:740.
Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet 1993;176:480–483.
Sainio V, Kemppaainen E, Puolakkainen P, et al. Early antibiotic treatment in acute necrotising pancreatitis. Lancet 1995;346:663–667.
Isaji S, Suzuki M, Frey CF, et al. Role of bacterial infection in diet-induced acute pancreatitis in mice. Int J Pancreatol 1992;11:49–57.
Lange JF, van Gool J, Tytgat GN. The protective effect of a reduction in intestinal flora on mortality of acute hemor-rhagic pancreatitis in the rat. Hepatogastroenterology 1987; 34:28–30.
Luiten EJT, Hop WCJ, Lange JF, Bruining HA. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg 1995;222:57–65.
Foitzik T, Stufler M, Hotz HG, et al. Glutamine stabilizes intestinal permeability and reduces pancreatic infection in acute experimental pancreatitis. J GASTROINTEST Surg 1997;1:40–47.
Gennari R, Alexander W. Effects of hyperoxia on bacterial translocation and mortality during gut-derived sepsis. Arch Surg 1996;131:57–62.
Simsek I, Mas MR, Yasar M, et al. Inhibition of inducible nitric oxide synthase reduces bacterial translocation in a rat model of acute pancreatitis. Pancreas 2002;23:296–301.
Czako I, Takacs T, Varga IS, et al. Oxidative stress in distant organs and the effects of allopurinol during experimental acute pancreatitis. Int J Pancreatol 2000;27:209–216.
Defaux JP, Thonier F, Baroggi N, et al. Involvement of platelet-activating factor (PAF) in endotoxinor ischaemia-induced intestinal hyperpermeability in the rat. J Lipid Me-diat 1993;7:11–21.
de Souza LJ, Sampietre SN, Assis RS, et al. Effect of PAF antagonists (BN-52021, WEB-2170, and BB-882) on bacterial translocation in acute pancreatitis. J GASTROINTEST Surg 2001;5:364–370.
Sun Z, Wang X, Deng X, et al. Beneficial effects of lexi-pafant, a PAF antagonist, on gut barrier dysfunction caused by intestinal ischaemia and reperfusion in rats. Dig Surg 2000;17:57–60.
Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut 2001;48:62–69.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rahman, S.H., Ammori, B.J., Holmfield, J. et al. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 7, 26–36 (2003). https://doi.org/10.1016/S1091-255X(02)00090-2
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1091-255X(02)00090-2