Abstract
Objective
Pronounced dilation of the maternal vasculature occurs during normal pregnancy. Likewise, low resistance characterizes the fetoplacental circulation. Nitric oxide released by endothelial cells is a potent vasodilator known to be a key modulator of both maternal and fetal vascular tone. However, the mechanisms underlying the maternal circulatory adaptations and the low resistance of the fetal circulation remain unknown. The aim of the present study was to compare levels of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase, in the maternal and fetal circulations.
Methods
High performance liquid chromatography was used to measure asymmetric ADMA in the maternal and umbilical venous plasma.
Results
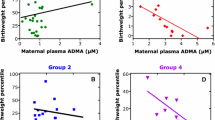
Plasma ADMA levels during the first trimester were 0.29 ± 0.05, the third trimester before term 0.29 ± 0.05, and at term 0.32 ± 0.05 nmol/mL, which were significantly (P <.05) lower than the levels measured in nonpregnant women (0.41 ± 0.06 nmol/mL). By contrast, ADMA levels in umbilical venous plasma averaged 1.02 ± 0.18 nmol/mL, significantly (P <.005) higher than maternal levels. Unlike ADMA, levels of plasma l-arginine, the nitric oxide precursor, did not significantly differ among nonpregnant and pregnant women and the fetus.
Conclusion
During pregnancy, maternal hemodynamics are modulated, at least in part, by a reduction in ADMA. Conversely, the low resistance to umbilical blood flow is maintained despite substantially higher fetal ADMA levels. Thus, the predominant mechanisms regulating the maternal and fetal circulation apparently differ.
Similar content being viewed by others
References
Rosenfeld CR. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am J Physiol 2001;281:R1025–40.
Yoshimura T, Magness RR, Rosenfeld CR. Angiotensin II and α-agonist. I. Responses of ovine fetoplacental vasculature. Am J Physiol 1990;259:H464–72.
Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol 1997;272:R441–63.
Williams DJ, Vallance PJT, Neild GH, Spencer JAD, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol 1997;272:H748–52.
Young D, Lang U, Greenberg SG, Myatt L, Clark KE. Elevation of nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol 1996;174:573–7.
Böger RH, Bode-Böger SM. Asymmetrie dimethylarginine, derangements of the endothelial nitric oxide synthase pathway, and cardiovascular diseases. Semin Thromb Hemost 2000;26:539–45.
Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol 2000;20:2032–7.
Fickling SA, Leone AM, Moncada S, Nussey SS, Vallance P, Whitley GS. Synthesis of NG,NG dimethylarginine by human endothelial cells. Endothelium 1993;1:137–40.
Matsuoka H, Itoh S, Kimoto M, et al. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension 1997;29(Part 2):242–7.
Yoshimura T, Yoshimura M, Tabata A, et al. Association of missense Glu298Asp variant of the endothelial nitric oxide synthase gene with severe preeclampsia. J Soc Gynecol Investig 2000;7:238–41.
Holden DP, Fickling SA, Whitley GS, Nussey SS. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am J Obstet Gynecol 1998;178:551–6.
Petterson A, Hedner T, Milson I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet Gynaecol Scand 1998;77:808–13.
Ellis J, Wennerholm UB, Bengtsson A, et al. Levels of dimethy-larginines and cytokines in mild and severe preeclampsia. Acta Obstet Gynaecol Scand 2001;80:602–8.
Myatt L, Eis AL, Broekman DE, Greer IA, Lyall F. Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum Reprod 1997;12:167–72.
Desrosiers R, Tanguay RM. Methylation of Drosphilia histones at proline, lysine and arginine residues during heat shock. J Biol Chem 1988;263:4686–92.
Wang C, Lin JM, Lazarides E. Methylations of 70,000-Da heat shock proteins in 3T3 cells: Alterations by arsenite treatment, by different stages of growth and by virus transformation. Arch Biochem Biophys 1992;297:169–75.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, T., Yoshimura, T. & Okamura, H. Asymmetric Dimethylarginine, an Endogenous Inhibitor of Nitric Oxide Synthase, in Maternal and Fetal Circulation. Reprod. Sci. 10, 2–4 (2003). https://doi.org/10.1016/S1071-5576(02)00192-2
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1071-5576(02)00192-2