Abstract
Background
Monoclonal antibodies are attractive agents for noninvasive localization of various cardiovascular disorders. Because proliferating intimal smooth muscle cells are important components of atherosclerotic lesions, radiolabeled antibody Z2D3 specific for proliferating smooth muscle cells has been used for immunoscintigraphic localization of experimental atherosclerotic lesions. This study was undertaken to assess the role of antibody affinity in optimization of immunoscintigraphic localization of these lesions. Z2D3 belongs to the immunoglobulin (Ig) M class of antibodies. For immunoscintigraphic studies attempts were made to prepare F(ab')2 or Fab fragments from the parent cell line. Fragmentation of Z2D3-IgM or its two subclones (B7 and 2B12) was not possible; therefore the parent hybridoma line was subjected to class switching. Cell lines 5C5 and 3E5 secreted antibody of the IgG1 subclass. The Z2D3-IgG1 antibodies were enzymatically digested to provide F(ab')2. Because of a tenfold loss of immunoreactivity of these class-switched antibodies, the parent clone was subsequently genetically engineered to obtain a mouse/human chimeric antibody with human IgG1 constant region. F(ab')2 of Z2D3-73.30 chimeric antibody retained the immunoreactivity relative to the original Z2D3-IgM. Radiolabeled murine and chimeric F(ab')2 fragments were used to assess the role of affinity in gamma scintigraphic visualization of experimental atherosclerotic lesions.
Methods and Results
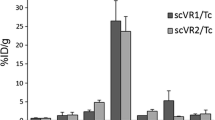
Experimental atherosclerotic lesions were induced in 19 rabbits by abdominal aortic balloon deendothelialization followed by a hyperlipidemic diet for 12 weeks. 111In-labeled chimeric high-affinity Z2D3 F(ab')2 fragments (111In-Hi.aff Z2D3) were administered in four animals. Uptake was compared with 111In-labeled F(ab')2 of nonspecific human IgG1 (n=4), murine low-affinity Z2D3-5C5 (111In-Lo.aff Z2D3; n=4), nonspecific murine IgG1 monoclonal antibodies (n=4) and 123I-labeled murine low-affinity Z2D3-3E5 (n=3). Atherosclerotic lesions were visualized 48 hours after administration of the chimeric Hi.aff-Z2D3 antibody in all animals. Lesions were not visualized in rabbits injected with Lo.aff-Z2D3 or murine or human nonspecific antibodies. Mean percent injected dose per gram in the lesion was significantly higher with the 111In-Hi.aff-Z2D3 (0.112%±0.024%) compared with 111In-Lo.aff-Z2D3 (0.037%±0.005%; p=0.03), human nonspecific (0.027%±0.004%; p=0.01) or murine nonspecific antibodies (0.006%±0.001%; p=0.0004). Nonspecific activity in unballooned thoracic aortic segments (normal) was comparable in the 111In-Hi.aff-Z2D3 (0.019±0.003) and the 111In-Lo.aff-Z2D3 (0.011%±0.005%; p=0.3) antibodies. The lesion activities of the Lo.aff-Z2D3 labeled with 111In (0.037±0.005) or 123I (0.034±0.007; p=0.71) were similar regardless of the radioisotopes used for labeling.
Conclusions
Our study demonstrates that the specificity of an antibody for the target antigen in the atheroma is a necessary condition for in vivo targeting. However, high enough affinity of an antibody is an essential component for noninvasive diagnostic visualization of experimental atherosclerotic lesions.
Similar content being viewed by others
References
Khaw BA, Narula J, Nicol PD. Myocyte necrosis-avid imaging with radiolabeled antimyosin antibody: experimental and clinical acute myocardial infarction, myocarditis and heart transplant rejection. In: van der Wall EE Sochor H, Righetti A, Niemeyer MG, eds. What's new in cardiac imaging? SPECT, PET and MRI. Dordretcht, The Netherlands: Kluwer, 1992:295–313.
Fischman AJ, Khaw BA, Strauss HW. Quo vadis: radioimmune imaging. J Nucl Med 1989;30:1911–3.
Harrison DC, Calenoff E, Chen FW, Parmley WW, Khaw BA, Ross R. Plaque associated immune reactivity as a tool for the diagnosis and treatment of atherosclerosis. Proceedings of the American Clinical and Climabiological Association, 1991:210–7.
Dietrich DR. Toxicological and pathological applications of proliferating cell nuclear antigen (PCNA): a novel endogenous marker for cell proliferation. Crit Rev Toxicol 1993;23:77–109.
Kocher O, Gabbiani F, Gabbiani G, et al. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening: biochemical and morphologic studies. Lab Invest 1991;65:459–70.
Narula J, Petrov A, Bianchi C, et al. Noninvasive localization of experimental atherosclerotic lesions with mouse/human chimeric antibody Z2D3 specific for proliferating smooth muscle cells in human atheroma: imaging with conventional antibody and with negative charge modified antibody fragments. Circulation 1995; 92:474–84.
Kabat EA, Wu TT, Reid-Miller M, Perry HM, Gottesman KS. Sequences of proteins of immunological interest. US Department of Health and Human Services, 1987.
Orlandi R, Gussow DH, Jones PT, Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci USA 1989;86:3833–7.
Takahashi N, Ueda S, Obata M, Nikaido T, Nakai S, Honjo T. Structure of human immunoglobulin gamma genes: implications for evolution of a gene family. Cell 1982;29:671–9.
Heiter PA, Max EE, Seidman JG, Maizel JV Jr Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell 1980;22:197–207.
Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol 1982;93:576–82.
Hnatowich DL, Layne WW, Childs RL, et al. Radioactive labeling of antibody: a simple and efficient method. Science 1983;220:613–5.
Khaw BA, Strauss HW, Moore R et al. Myocardial damage delineated by indium-111 antimyosin Fab and technetium-99m pyrophosphate. J Nucl Med 1987;28:76–82.
Hunter WM, Greenwood FC. Preparation of I-131 labeled human growth hoemone of high specific activity. Nature 1962;194:495–6.
Baumgartener HR. Eine neue Methode zur Erxeugung von Thromben durch geziette uberdehnung der, Gefasswand. Z Gesamte Exp Med 1963;137:227–49.
RS/1 Release Software. BBN Software Product Corp, 1988.
Thyberg J, Heidin U, Sjolund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smoth muscle cells. Arteriosclerosis 1990;10:966–84.
Raines EW, Ross R Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J 1993;69:S30–7.
Grotendorst GR, Seppa HEJ, Kleinman HK, Martin GR. Attachment of smooth muscle cells to collagen and their migration toward platelet growth factor. Proc Natl Sci USA 1981;78:3669–72.
Pieri P, Carrio I, Narula J et al. Imaging of human atherosclerotic lesions with indium-111-labeled Z2D3 antibody specific for proliferating smooth muscle cells of human atheroma. [Abstract]. J Am Coll Cardiol 1996;27:321.
Narula J, Petrov A, Ditlow C, Chen F, Khaw BA. Localization of atherosclerotic lesions with indium-111-chimeric Z2D3 antibody in Watanabe rabbits and rabbits with experimentally-induced lesions [Abstract]. J Nucl Cardiol 1995;2:S112.
Khaw BA, Strauss HW, Narula J. Magic bullets: from muskets to smart bombs. J Nucl Med 1993;34:2264–8.
Hnatowich DJ, Fritz B, Virzi F, Mardirossian G, Rusckowski M. Improved tumor localization with (strep)avid and labeled biotin as a substitute for antibody. Nucl Med Biol 1993;20:189–95.
Le Doussal JM, Gruaz-Guyon A, Martin M, Gautherot E, Delaage M, Barbet J. Targeting of indium-111-labeled bivalent hapten to human melanoma mediated by bispecific monoclonal antibody conjugates: imaging of tumors hosted in nude mice. Cancer Res 1990;50:3445–52.
Khaw BA, Klibanov A, O'Donnell SM, et al. Gamma imaging with negatively charge-modified monoclonal antibody: modification with synthetic polymers. J Nucl Med 1991;32:1742–51.
Eichmann K, Lackland H, Hood L, Krause RM. Induction of rabbit antibody with molecular uniformity after immunization with group C streptococci. J Exp Med 1970;131:207–21.
Levine S, Levine M, Sharp KA, Brooks DE. Theory of electrokinetic behavior of human erythrocytes. Biophys J 1983;42:127–35.
Gallagher JE, George G, Brody AE. Sialic acid mediates the initial binding of positively charged inorganic particles to alveolar macrophage membranes. Ann Rev Respir Dis 1987;135:1345–52.
Silva Filho HC, Santos ABS, de Carvelho TMU, DeSouza W. Surface charge of resident, elicited and activated mouse peritoneal macrophages. J Leukoc Biol 1987;41:143–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Narula, J., Petrov, A., O'Donnell, S.M. et al. Gamma imaging of atherosclerotic lesions: The role of antibody affinity in in vivo target localization. J Nucl Cardiol 3, 231–241 (1996). https://doi.org/10.1016/S1071-3581(96)90037-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1071-3581(96)90037-9