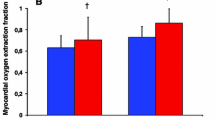
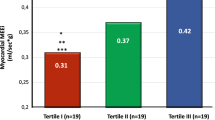
Abstract
Background
The effects of exercise training on myocardial substrate utilization have not previously been studied in patients with idiopathic dilated cardiomyopathy and mild heart failure.>/<
Methods and Results
Myocardial glucose uptake was studied in 15 clinically stable patients with dilated cardiomyopathy (New York Heart Association class I-II, ejection fraction 34% ± 8%) with the use of 2-[fluorine 18]fluoro-2-deoxy-D-glucose ([F-18]FDG) and positron emission tomography under euglycemic hyperinsulinemia. Eight of these patients participated in a 5-month endurance and strength training program, whereas seven patients served as nontrained subjects. Left ventricular function was assessed by 2-dimensional echocardiography before and after the intervention. After the training period, insulin-stimulated myocardial fractional [F-18]FDG uptake and glucose uptake rates were significantly increased in the anterior, lateral, and septal walls (P > .01) in the trained subjects but remained unchanged in the nontrained subjects. In the trained patients, whole-body insulin-stimulated glucose uptake was enhanced and serum free fatty acid levels were suppressed during hyperinsulinemia compared with the baseline study (P > .05). No changes were observed in the nontrained group.>/<
Conclusions
These results indicate that exercise training in patients with dilated cardiomyopathy improves insulin-stimulated myocardial glucose uptake. This improvement in glucose uptake may be indicative of a switch in myocardial preference to a more energy-efficient substrate. >/<
Similar content being viewed by others
References
Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173–82.
Delagardelle C, Feiereisen P, Krecke R, Essamri B, Beissel J. Objective effects of a 6 months' endurance and strength training program in outpatients with congestive heart failure. Med Sci Sports Exerc 1999;31:1102–7.
Hambrecht R, Fiehn E, Weigl C, et al Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998;98:2709–15.
Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 1988;78:506–15.
Hare DL, Ryan TM, Selig SE, et al Resistance exercise training increases muscle strength, endurance, and blood flow in patients with chronic heart failure. Am J Cardiol 1999;83:1674–7, A7.
Magnusson G, Gordon A, Kaijser L, et al High intensity knee extensor training, in patients with chronic heart failure. Major skeletal muscle improvement. Eur Heart J 1996;17:1048–55.
Maiorana A, O'Driscoll G, Cheetham C, et al Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. J Appl Physiol 2000;88:1565–70.
Linke A, Schoene N, Gielen S, et al Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol 2001;37:392–7.
Hambrecht R, Fiehn E, Yu J, et al Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 1997;29:1067–73.
Bengel FM, Permanetter B, Ungerer M, Nekolla S, Schwaiger M. Non-invasive estimation of myocardial efficiency using positron emission tomography and carbon-11 acetate-comparison between the normal and failing human heart. Eur J Nucl Med 2000;27:319–26.
Davila-Roman VG, Vedala G, Herrero P, et al Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271–7.
Paternostro G, Camici PG, Lammerstma AA, et al Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest 1996;98:2094–9.
Taylor M, Wallhaus TR, DeGrado TR, et al An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in patients with congestive heart failure. J Nucl Med 2001;42:55–62.
Kainulainen H, Virtanen P, Ruskoaho H, Takala TE. Training increases cardiac glucose uptake during rest and exercise in rats. Am J Physiol 1989;257:H839–45.
Kainulainen H, Komulainen J, Takala T, Vihko V. Effect of chronic exercise on glucose uptake and activities of glycolytic enzymes measured regionally in rat heart. Basic Res Cardiol 1989;84:174–90.
Martineau LC, Chadan SG, Parkhouse WS. Age-associated alterations in cardiac and skeletal muscle glucose transporters, insulin and IGF-1 receptors, and PI3-kinase protein contents in the C57BL/6 mouse. Mech Ageing Dev 1999;106:217–32.
Martineau LC, Chadan SG, Parkhouse WS. Resistance of the aged myocardium to exercise-induced chronic changes in glucose transport related protein content. Mech Ageing Dev 1999;110:109–18.
Stuewe SR, Gwirtz PA, Agarwal N, Mallet RT. Exercise training enhances glycolytic and oxidative enzymes in canine ventricular myocardium. J Mol Cell Cardiol 2000;32:903–13.
Stolen KQ, Kemppainen J, Ukkonen H, et al Exercise training improves biventricular oxidative metabolism and left ventricular efficiency in patients with dilated cardiomyopathy. J Am Coll Cardiol 2003;41:460–7.
Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 1983;15:523–8.
Jackson A, Pollock M. Practical assessment of body composition. Phys Sportsmed 1985;13:76–90.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–23.
Nuutila P, Peltoniemi P, Oikonen V, et al Enhanced stimulation of glucose uptake by insulin increases exercise-stimulated glucose uptake in skeletal muscle in humans: studies using [15O]O2, [15O]H2O, [18F]fluoro-deoxy-glucose, and positron emission tomography. Diabetes 2000;49:1084–91.
Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 1986;27:235–8.
Alenius S, Ruotsalainen U. Bayesian image reconstruction for emission tomography based on median root prior. Eur J Nucl Med 1997;24:258–65.
Schelbert H, Schwaiger M. Positron emission tomography and autoradiography: principles and applications for the brain and heart. In: Phelps M, Marizziotta J, Schelbert H, editors. PET studies of the heart. New York: Raven Press; 1986. p. 599–616.
Sokoloff L, Reivich M, Kennedy C, et al The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977;28:897–916.
Ng CK, Soufer R, McNulty PH. Effect of hyperinsulinemia on myocardial fluorine-18-FDG uptake. J Nucl Med 1998;39:379–83.
Stolen K, Kemppainen J, Kalliokoski K, et al Left and right ventricular oxidative metabolism in patients with mild heart failure [abstract]. Eur J Heart Fail Suppl 2002;1:S39.
Paolisso G, Gambardella A, Galzerano D, et al Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism 1994;43:174–9.
Opie L. The heart physiology, from cell to circulation. 3rd ed. Philadelphia: Lippincott-Raven; 1998.
Beanlands RS, Nahmias C, Gordon E, et al The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: a double-blind, placebo-controlled, positron-emission tomography study. Circulation 2000;102:2070–5.
Wallhaus TR, Taylor M, DeGrado TR, et al Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 2001;103:2441–6.
Eichhorn EJ, Heesch CM, Barnett JH, et al Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double-blind, placebocontrolled study. J Am Coll Cardiol 1994;24:1310–20.
Knuuti MJ, Maki M, Yki-Jarvinen H, et al The effect of insulin and FFA on myocardial glucose uptake. J Mol Cell Cardiol 1995;27:1359–67.
Nuutila P, Knuuti MJ, Raitakari M, et al Effect of antilipolysis on heart and skeletal muscle glucose uptake in overnight fasted humans. Am J Physiol 1994;267:E941–6.
Pietila M, Malminiemi K, Vesalainen R, et al Exercise training in chronic heart failure: beneficial effects on cardiac (11)C-hydroxyephedrine PET, autonomic nervous control, and ventricular repolarization. J Nucl Med 2002;43:773–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported in part by the Ministry of Education, the Aarne Koskelo Foundation, the Research Foundation of Orion Corporation, and the Finnish Cardiovascular Foundation.
Rights and permissions
About this article
Cite this article
Stolen, K.Q., Kemppainen, J., Kalliokoski, K.K. et al. Exercise training improves insulin-stimulated myocardial glucose uptake in patients with dilated cardiomyopathy. J Nucl Cardiol 10, 447–455 (2003). https://doi.org/10.1016/S1071-3581(03)00528-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1071-3581(03)00528-2