Abstract
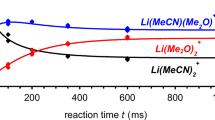
Analysis of the sites of reaction of a biologically important compound, pilocarpine, a molecule with imidazole and butyrolactone rings connected by a methylene bridge, has been accomplished in a quadrupole ion trap with the aim of characterizing its structure/reactivity relationships. Ion-molecule reactions of pilocarpine with chemical ionizing agents, dimethyl ether (DME), 2-methoxyethanol, and trimethyl borate (TMB), along with collision-activated dissociation elucidated the reaction sites of pilocarpine and made possible the comparison of structural features that affect sites of reaction. Based on MS/MS experiments, methylation occurs on the imidazole ring upon reactions with CH3OCH +2 or (CH3OCH2CH2OH)H+ ions but methylation occurs on the lactone ring for reactions with (CH3O)2B+ ions. Bracketing experiments with two model compounds, α-methyl-γ-butyrolactone and N-methyl imidazole, show the imidazole ring to have a greater gas-phase basicity and methyl cation affinity than the lactone ring. The contrast of methylation by TMB ions on the lactone ring is explained by initial addition of the dimethoxyborinium ion, (CH3O)2B+, on the imidazole ring with subsequent collisional activation promoting an intramolecular transfer of a methyl group to the lactone ring with concurrent loss of CH3OBO. Semiempirical molecular orbital calculations are undertaken to further address the favored reaction sites.
Similar content being viewed by others
References
Alvarez, E. J.; Brodbelt, J. S. Selective Ion-Molecule Reactions of Ether Reagent Ions with Nucleoside Antibiotics in a Quadrupole Ion Trap. J. Am. Soc. Mass Spectrom. 1995, 30, 625–631.
Luna, A.; Amekraz, B.; Morizur, J.-P.; Tortajada, J.; Mo, O.; Yanez, M. Reactions between Guanidine and Cu+ in the Gas Phase. An Experimental and Theoretical Study. J. Phys. Chem. A 1997, 101, 5931–5941.
Camara, E.; Green, M. K.; Penn, S. G.; Lebrilla, C. B. Chiral Recognition is Observed in the Deprotonation Reaction of Cytochrome c by (2R)- and (2S)-2-Butylamine. J. Am. Chem. Soc. 1996, 118, 8751–8752.
Stephenson, J. L.; McLuckey, S. A. Ion/Ion Proton Transfer Reactions for Protein Mixture Analysis. Anal. Chem. 1996, 68, 4026–4032.
Alvarez, E. J.; Vartanian, V. H.; Brodbelt, J. S. Metal Complexation Reactions of Quinolone Antibiotics in a Quadrupole Ion Trap. Anal. Chem. 1997, 69, 1147–1155.
Colorado, A.; Brodbelt, J. Class-Selective Collisionally Activated Dissociation/Ion-Molecule Reactions of 4-Quinolone Antibiotics. Anal. Chem. 1994, 66, 2330–2335.
Alvarez, E. J.; Brodbelt, J. S. Evaluation of Metal Complexation as an Alternative to Protonation for Electrospray Ionization of Pharmaceutical Compounds. J. Am. Soc. Mass Spectrom. 1998, 9, 463–472.
Orlando, R.; Murphy, C.; Fenselau, C.; Hansen, G.; Cotter, R. J. Endothermic Ion-Molecule Reactions: Strategies for Tandem Mass Spectrometric Structural Analyses of Large Biomolecules. Anal. Chem. 1990, 62, 125–129.
Orlando, R.; Fenselau, C.; Cotter, R. J. Endothermic Ion-Molecule Reactions. 4. Site-Directed Fragmentation in N-Acetylated Oligosaccharides at Low Beam Energies. Anal. Chem. 1990, 62, 2388–2390.
Dongre’, A. R.; Jones, J. L.; Somogyi, A.; Wysocki, V. H. Influence of Peptide Composition, Gas-Phase Basicity, and Chemical Modification on Fragmentation Efficiency. Evidence for the Mobile Proton Model. J. Am. Chem. Soc. 1996, 118, 8365–8374.
Cheng, X.; Bakhtiar, R.; Van Orden, S.; Smith, R. D. Charge-State Shifting of Individual Multiply-Charged Ions of Bovine Albumin Dimer and Molecular Weight Determination Using an Individual-Ion Approach. Anal. Chem. 1994, 66, 2084–2087.
Gross, D. S.; Williams, E. R. Experimental Measurement of Coulomb Energy and Intrisic Dielectric Polarizability of a Multiply Protonated Peptide Ion Using Electrospray Ionization Fourier-Transform Mass Spectrometry. J. Am. Chem. Soc. 1995, 117, 883–890.
Winger, B. E.; Light-Wahl, K. J.; Rockwood, A. L.; Smith, R. D. Probing Qualitative Conformation Differences of Multiply Protonated Gas-Phase Proteins via H/D Isotopic Exchange with D2O. J. Am. Chem. Soc. 1992, 114, 5897–5898.
Gard, E.; Kirk Green, M.; Bregar, J.; Lebrilla, C. B. Gas-Phase Hydrogen/Deuterium Exchange as a Molecular Probe for the Interaction of Methanol and Protonated Peptides. J. Am. Soc. Mass Spectrom. 1994, 5, 623–631.
Wood, T. D.; Chorush, R. A.; Wampler, F. M. III; Little, D. P.; O’Connor, P. B.; McLafferty, F. W. Gas-phase folding and unfolding of cytochrome c cations. Proc. Natl. Acad. Sci. USA 1995, 92, 2451–2454.
Campbell, S.; Rodgers, M. T.; Marzluff, E. M.; Beauchamp, J. L. Deuterium Exchange Reactions as a Probe of Biomolecule Structure. Fundamental Studies of Gas Phase H/D Exchange Reactions of Protonated Glycine Oligomers with D2O, CD3OD, CD3CO2D, and ND3. J. Am. Chem. Soc. 1995, 117, 12840–12854.
Gur, E. H.; deKoning, L. J.; Nibbering, N. M. M. The Bimolecular Hydrogen-Deuterium Exchange Behavior of Protonated Alkyl Dipeptides in the Gas Phase. J. Am. Soc. Mass Spectrom. 1995, 6, 466–477.
Goodman and Gilman’s The Pharmacological Basis of Therapeutics Ninth Edition; Hardman, J. G.; Limbird, L. E.; Goodman Gilman, A., Eds.; McGraw-Hill: New York, 1996; p 146.
Brodbelt, J.; Liou, C. C.; Donovan, T. Selective Adduct Formation by Dimethyl Ether Chemical Ionization in a Quadrupole Ion Trap Mass Spectrometer and a Conventional Ion Source. Anal. Chem. 1991, 63, 1205–1209.
Keough, T. Dimethyl Ether as a Reagent Gas for Organic Functional Group Determination by Chemical Ionization Mass Spectrometry. Anal. Chem. 1982, 54, 2540–2547.
Burrows, E. Dimethyl Ether Chemical Ionization Mass Spectrometry. Mass. Spec. Rev. 1995, 14, 107–115.
Suming, H.; Yaozu, C.; Longfei, J.; Shuman, X. Stereochemical Effects in Mass Spectrometry. 2-Chemical Ionization Mass Spectra of Some Cyclic Glycols and Mono- and Disaccharides Using Trimethyl Borate as Reagent Gas. Org. Mass Spectrom. 1985, 20, 719–723.
Leeck, D. T.; Ranatunga, T. D.; Smith, R. L.; Partanen, T.; Vainiotalo, P.; Kenttamaa, H. I. Differentiation of stereoisomeric diols by using CH3OB+OCH3 in a small Fourier transform ion cyclotron resonance mass spectrometer. Int. J. Mass Spectrom. Ion Processes 1995, 141, 229–240.
Colorado, A.; Brodbelt, J. Borinium Adduct Ion Formation with Barbiturates in a Quadrupole Ion-trap Mass Spectrometer. J. Mass Spectrom. 1996, 31, 403–410.
Kempen, E. C.; Brodbelt, J. Use of Trimethyl Borate as a Chemical Ionization Reagent for the Analysis of Biologically Active Molecules. J. Mass Spectrom. 1997, 32, 846–854.
Bauerle, G. F.; Hall, B. J.; Tran, N. V.; Brodbelt, J. Ion-Molecule Reactions of Oxygenated Chemical Ionization Reagents with Vincamine. J. Am. Soc. Mass Spectrom. 1995, 7, 250–260.
Alvarez, E. J.; Brodbelt, J. S. Selective Ion-Molecule Reactions of Ether Reagent Ions with Nucleoside Antibiotics in a Quadrupole Ion Trap. J. Mass Spectrom. 1995, 30, 625–631.
Hunter, E. P. and Lias, S. G. Proton Affinity Evaluation In NIST Standard Reference Database Number 69, Mallard, W. G.; Lindstrom, P. J. Eds.; National Institute of Standards and Technology: Gaithersburg, MD, August 1997 (http://webbook.nist.gov).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satterfield, M., Brodbelt, J.S. Sites of reaction of pilocarpine. J Am Soc Mass Spectrom 10, 209–216 (1999). https://doi.org/10.1016/S1044-0305(98)00141-X
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(98)00141-X