Abstract
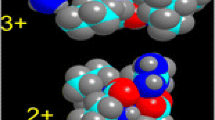
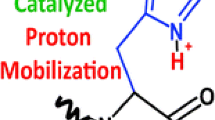
A model was developed to describe the deuterium uptake of gas phase polypeptide ions via H/D exchange with D2O. Ab initio calculations established, for energetic reasons, that the exchange must take place via a “relay” mechanism involving both a charged site and a nearby basic site. Molecular dynamics simulations indicated that the D2O molecule did not penetrate the core of the example peptide, protonated bradykinin (Bk+H)+, and hence the relay mechanism must occur on the peptide surface. Two factors were deemed to be important: (1) The surface accessibility of the charged sites and the basic sites and (2) the distances between them. An algorithm was developed that accounted for these features using the absolute exchange rate as a free parameter. Excellent agreement was obtained with experiment when equal weight was given to an ensemble of low energy conformations of (Bk+H)+, assumed to have a salt bridge primary structure. Single conformations, or other protonated forms, did not allow good agreement with experiment for any value of the absolute exchange rate constant.
Similar content being viewed by others
References
Monaghan, J. J.; Barber, M.; Bordoli, R. S.; Sedgwick, R. D.; Tyler, A. N. Org. Mass Spectrom. 1983, 18, 75, 1982, 17, 569, 1982, 17, 529.
Hillenkamp, F.; Karas, M.; Beavis, R. C.; Chait, B. T. Anal. Chem. 1991, 63, A1193.
Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. Mass Spectrom. Rev. 1990, 9, 37.
Englander, S. W.; Kallenbach, N. R. Q. Rev. Biophys. 1983, 16, 521.
Smith, D. L.; Zhang, Z. Mass Spectrom. Rev. 1994, 13, 411.
Green, M. K. Lebrilla, C. B. Mass Spectrom. Rev. 1997, 16, 53.
Covey, T.; Douglas, D. J. J. Am. Soc. Mass Spectrom. 1993, 4, 616.
Cox, K. A.; Julian, R. K.; Cooks, R. G.; Kaiser, R. E. J. Am. Soc. Mass Spectrom. 1994, 5, 127.
Ganem, B.; Li, Y.-T.; Henion, J. D. J. Am. Chem. Soc. 1991, 113, 6294.
Cheng, X.; Chen, R.; Bruce, J. E.; Schwartz, B. L.; Anderson, G. A.; Hofstadler, S. A.; Gale, D. C.; Smith, R. D.; Gao, J.; Sigal, G. B.; Mammen, M.; Whitesides, G. M. J. Am. Chem. Soc. 1995, 117, 8859.
Clemmer, D. E.; Hudgins, R. R.; Jarrold, M. F. J. Am. Chem. Soc. 1995, 117, 10141.
von Helden, G.; Wyttenbach, T.; Bowers, M. T. Science 1995, 267, 1483.
Sullivan, P. A.; Axelsson, J.; Altmann, S.; Quist, A. P.; Sunqvist, B. U. R.; Reimann, C. T. J. Am. Soc. Mass Spectrom. 1996, 7, 329.
Gross, D. S.; Williams, E. R. J. Am. Chem. Soc. 1995, 117, 883.
Kaltashov, I. A.; Fenselau, C. Proteins: Structure Function Genetics 1997, 27, 165.
Dongre, A. R.; Somogyi, A.; Wysocki, V. H. J. Mass Spectrom. 1996, 31, 339.
Katta, V.; Chait, B. T. Rapid Commun. Mass Spectrom. 1991, 5, 214.
J. Am. Chem. Soc. 1993, 115, 6317.
Smith, D. L.; Deng, Y.; Zhang, Z. J. Mass Spectrom. 1997, 32, 135.
Miranker, A.; Robinson, C. V.; Radford, S. E.; Aplin, R. T.; Dobson, C. M. Science 1993, 262, 896.
Johnson, R. S.; Walsh, K. A. Prot. Sci. 1994, 3, 2411.
Wagner, D. S.; Melton, L. G.; Yan, Y.; Erickson, B. W.; Anderegg, R. J. Protein Sci. 1994, 3, 1305.
Winger, B. E.; Light-Wahl, K. J.; Rockwood, A. L.; Smith, R. D. J. Am. Chem. Soc. 1992, 114, 5897.
Cheng, X.; Fenselau, C. Int. J. Mass Spectrom. Ion Processes 1992, 122, 109.
Hemling, M. E.; Conboy, J. J.; Bean, M. F.; Mentzer, M.; Carr, S. A. J. Am. Soc. Mass Spectrom. 1994, 5, 434.
Wood, T. D.; Chorush, R. A.; Wampler, F. M., III; Little, D. P.; O’Connor, P. B.; McLafferty, F. W. Proc. Natl. Acad. Sci. USA 1995, 92, 2451.
Clemmer, D. E.; Valentine, S. J. J. Am. Chem. Soc. 1997, 119, 3558.
Zhang, X; Ewing, N; Cassady C. J. Int. J. Mass Spectrom. Ion Processes, submitted.
Kemper, P. R.; Bowers, M. T. J. Phys. Chem. 1991, 95, 5134.
Wyttenbach, T.; von Helden, G.; Bowers, M. T. J. Am. Chem. Soc. 1996, 118, 8355.
Zhang, Z.; Li, W.; Guan, S.; Marshall, A. G. Proceedings of the 44th ASMS Conference on Mass Spectrometry and Allied Topics; Portland, OR, 1996; p 1061.
Guan, S.; Kim, H. S.; Marshall, A. G.; Wahl, M. C.; Wood, T. D.; Xiang, X. Chem. Rev. 1994, 94, 2161.
Recent results obtained at the National High Magnetic Field Laboratory in Florida indicate that thermal (300 K) protonated bradykinin exposed to 10−5 torr D2O vapor does not exchange any hydrogens at all during the course of a 1 h experiment. Although this result is obtained under more controlled experimental conditions than those reported here, it is of course useless for obtaining any structural information or testing an H/D exchange model. Freitas, M. A.; Marshall, A. G., private communication.
Schnier, P. D.; Price, W. D.; Jokusch, R. A.; Williams, E. R. J. Am. Chem. Soc. 1996, 118, 7178.
Campbell, S.; Rodgers, M. T.; Marzluff, E. M.; Beauchamp, J. L. J. Am. Chem. Soc. 1995, 117, 12840.
Stewart, J. J. P. J. Comp. Chem. 1989, 10, 209.
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S. J.; Windus, T. L.; Dupuis, M.; Montgomery, J. A. J. Comp. Chem. 1993, 14, 1347.
gaussian 94, Revision C. 2, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Gill, P. M. W.; Jonson, B. G.; Robb, M. A.; Cheesman, J. R.; Keith, T.; Petersson, G. A.; Montgomery, J. A.; Raghavachari, K.; Al-Laham, M. A.; Zakrzewski, V. G.; Ortiz, J. V.; Foresman, J. B.; Cioslowski, J.; Stefanov, B. B.; Nanayakkara, A.; Challacombe, M.; Peng, C. Y.; Ayala, P. Y.; Chen, W.; Wong, M. W.; Andres, J. L.; Replogle, E. S.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Binkley, J. S.; Defrees, D. F.; Baker, J.; Stewart, J. P.; Head-Gordon, M.; gonzalez, C.; Pople, J. A.; Gaussian, Inc., Pittsburgh PA, 1995.
Pearlman, D. A.; Case, D. A.; Caldwell, J. C.; Seibel, G. L.; Singh, U. C.; Weiner, P.; Kollman, P. A. amber 4. 0, Unversity of California, San Francisco.
The dielectric constant is often chosen to be proportional to 1/r implying that the potential energy between two charges q 1 and q 2 separated by r is E POT(r) α q 1 q 2/r 2. See, e. g., [18].
von Helden, G.; Wyttenbach, T.; Bowers, M. T. Int. J. Mass Spectrom. Ion Processes 1995, 146/147, 349.
Lee, S.; Wyttenbach, T.; Bowers, M. T. Int. J. Mass Spectrom. Ion Processes 1997, 167, 605.
Wyttenbach, T.; Bushnell, J. E.; Bowers, M. T. J. Am. Chem. Soc. 1998, 120, 5098.
Gidden, J.; Jackson, A. T.; Sceivens, J. H.; Bowers, M. T. Int. J. Mass Spectrom. Ion Processes, submitted.
On the basis of results reported in [12] simulations have been carried out on salt bridge structures arg1H+-arg9H+-COO−.
Gard, E.; Green, M. K.; Bregar, J.; Lebrilla, C. B. J. Am. Soc. Mass Spectrom. 1994, 5, 623.
Marshall, A. G.; Zhang, Z., private communication.
In order to determine the deuterium incorporation as shown in Figure 3 and deconvolute the mass spectra from the 12C/13C isotope distributions the maximum entropy method has been applied: Zhan, Z.; Guan, S.; Marshall, A. G. J. Am. Soc. Mass Spectrom. 1997, 8, 659.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wyttenbach, T., Bowers, M.T. Gas phase conformations of biological molecules: the hydrogen/deuterium exchange mechanism. J Am Soc Mass Spectrom 10, 9–14 (1999). https://doi.org/10.1016/S1044-0305(98)00121-4
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(98)00121-4