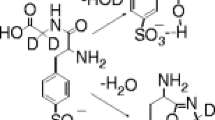
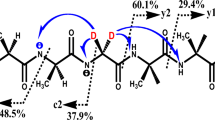
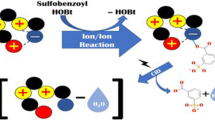
Abstract
Primary and secondary amines, when examined in atmospheric pressure chemical ionization, electrospray ionization, or chemical ionization, display protonated imines in their mass spectra. These products arise formally by nucleophilic substitution at the α-carbon with loss of both ammonia and molecular hydrogen. Collision-induced dissociation (CID) is used to characterize the product ions by comparison with authentic protonated imines. Gas-phase ion/molecule reactions of protonated amines with neutral amines also yield products that correspond to protonated imines (deamination and dehydrogenation), as well as providing simple deamination products. The reaction mechanism was investigated further by reacting the deamination product, the alkyl cation, with a neutral amine. The observed dehydrogenation of the nascent protonated secondary amine indicates that the reaction sequence is loss of ammonia followed by dehydrogenation even though the isolated protonated secondary amines did not undergo dehydrogenation upon CID. Formation of the deamination products in the protonated amine/amine reaction is competitive with proton-bound dimer formation. The proton-bound dimers do not yield deamination products under CID conditions in the ion trap or in experiments performed using a pentaquadrupole instrument. This demonstrates that the geometry of the proton-bound dimer, in which the α-carbons of the alkylamines are well separated [C a -N-H-N-C a ], is an unsuitable entry point on the potential energy hypersurface for formation of the imine [C a -N-C a ]. Isolation of the proton-bound dimers in the quadrupole ion trap is achieved with low efficiency and this characteristic can be used to distinguish them from their covalently bound isomers.
Similar content being viewed by others
References
Munson, M. S. B.; Field, F. H. J. Am. Chem. Soc. 1966, 88, 2621–2630.
Harrison, A. G. Chemical Ionization Mass Spectrometry; CRC: Boca Raton, FL, 1992.
Field, F. H. J. Am. Chem. Soc. 1970, 92, 2672–2676.
Herman, J. A.; Harrison, A. G. Can. J. Chem. 1981, 59, 2125–2131.
Jardine, I.; Catherine, F. J. Am. Chem. Soc. 1976, 98, 5086–5089.
Reiner, E. J.; Poirier, R. A.; Peterson, M. R.; Csizmadia, I. G.; Harrison, A. G. Can. J. Chem. 1986, 64, 1652–1660.
Sigsby, M. L.; Day, R. J.; Cooks, R. G. Org. Mass Spectrom. 1979, 14, 556–561.
Tedder, J. M.; Walker, G. S. J. Chem. Soc. Perkin Trans. 2 1991, 317–320.
Kaltashov, I. A.; Fenselau, C. C. Int. J. Mass Spectrom. Ion Processes 1995, 146/147, 339–347.
Li, X.; Harrison, A. G. Org. Mass Spectrom. 1993, 28, 366–371.
Cooks, R. G.; Kruger, T. L. J. Am. Chem. Soc. 1977, 99, 1279–1281.
Cooks, R. G.; Patrick, J. S.; Kotiaho, T.; McLuckey, S. A. Mass Spectrom. Rev. 1994, 13, 287–339.
Audier, H. E.; Millet, A.; Perret, C.; Tabet, J.-C.; Varenne, P. Org. Mass Spectrom. 1978, 13, 315–318.
Davis, D. V.; Cooks, R. G. Org. Mass Spectrom. 1981, 16, 176–179.
Wysocki, V. H.; Burinsky, D. J.; Cooks, R. G. J. Org. Chem. 1985, 50, 1287–1291.
Milne, G. W. A.; Axenrod, T.; Fales, H. M. J. Am. Chem. Soc. 1970, 92, 5170–5175.
Cleven, C. D.; Hoke, S. H.; Cooks, R. G.; Hrovat, D. A.; Smith, J. M.; Lee, M. S.; Borden, W. T. J. Am. Chem. Soc. 1996, 118, 10872–10878.
Brodbelt-Lustig, J. S.; Cooks, R. G. Talanta 1989, 36, 255–260.
Nourse, B. D.; Cooks, R. G. Int. J. Mass Spectrom. Ion Processes 1991, 106, 249–272.
Schwartz, J. C.; Schey, K. L.; Cooks, R. G. Int. J. Mass Spectrom. Ion Processes 1990, 101, 1–20.
Cooks, R. G.; Rockwood, A. L. Rapid Commun. Mass Spectrom. 1991, 5, 93.
Schwartz, J. C.; Wade, A. P.; Enke, C. G.; Cooks, R. G. Anal. Chem. 1990, 62, 1809–1818.
Hammerum, S.; Kuck, D.; Derrick, P. J. Tetrahedron Lett 1984, 25, 893–896.
Bowen, R. D. Mass Spectrom. Rev. 1991, 10, 225–279.
Gross, J. H.; Veith, H. H. Org. Mass Spectrom. 1993, 28, 867–872.
Louris, J. N.; Cooks, R. G.; Syka, J. E. P.; Kelly, P. E.; Stafford, G. C.; Todd, J. F. J. Anal. Chem. 1987, 59, 1677–1685.
March, R. E.; Todd, J. F. J. Practical Aspects of Ion Trap Mass Spectrometry, Vol. I: Fundamentals of Ion Trap Mass Spectrometry; CRC: Boca Raton, FL, 1995.
Vekey, K. J. Mass Spectrom. 1996, 31, 445–463.
Cox, K. A.; Cleven, C. D.; Cooks, R. G. Int. J. Mass Spectrom. Ion Processes 1995, 144, 47–65.
Mo, W.; Todd, J. F. J. Rapid Commun. Mass Spectrom. 1996, 10, 424–428.
Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. Mass Spectrom. Rev. 1990, 9, 37–70.
Bruins, A. P. Mass Spectrom. Rev. 1991, 10, 53–77.
Iraqui, M.; Lifshitz, C. Int. J. Mass Spectrom. Ion Processes 1989, 88, 45–57.
Audier, H. E.; Fossey, J.; Leblanc, D.; Mourgues, P. Bull. Soc. Chim. Fr. 1996, 133, 59–64.
Hunter, E. P.; Lias, S. G. J. Phys. Chem. Ref. Data to be published.
Mallard, W. G.; Linstrom, P. J. NIST Chemistry WebBook, NIST Standard Reference Database, Number 69; National Institute of Standards and Technology: Gaithersburg, MD 20899 (http//webbook. nist. gov), 1998.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, D., Gill, L.A. & Cooks, R.G. Deamination of protonated amines to yield protonated imines. J Am Soc Mass Spectrom 9, 1146–1157 (1998). https://doi.org/10.1016/S1044-0305(98)00093-2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(98)00093-2