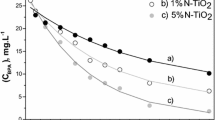
Abstract
The photocatalytic transformation of dexamethasone and the formation of its intermediate compounds have been studied using titanium dioxide as a photocatalyst. The degradation of dexamethasone occurs easily through the formation of several hydroxy derivatives whose characterization has been made by HPLC/MS/MS. Even if both oxidative and reductive processes can be operating, only oxidative products have been identified in air saturated aqueous suspensions. A pattern of reaction pathways accounting for the observed intermediates is proposed. The obtained experimental evidence may be rationalized postulating the existence of a double initial mechanism. A single oxidation step resulting from the attack by one ·OH radical leading to the formation of five hydroxy-derivatives and a concomitant attack involving two ·OH radicals leading to the hydroxylation of the quinoid moiety of the molecule.
Similar content being viewed by others
References
Gentile, D. M.; Tomlinson, E. S.; Maggs, J. L.; Park, B. K.; Back, D. J. J. Pharmacol. Exp. Ther. 1996, 277(1), 105–112.
Tomlinson, E. S.; Maggs, J. L.; Park, B. K.; Back, D. J. J. Steroid Biochem. 1997, 62(4), 345–352.
Tomlinson, E. S.; Lewis, D. F. V.; Maggs, J. L.; Kroemer, H. K.; Park, B. K.; Back, D. J. Biochem. Pharmacol. 1997, 54(5), 605–611.
Pruden, A. L.; Ollis, D. Environ. Sci. Technol. 1983, 17, 628.
Hsiao, C. Y.; Lee, C. L.; Ollis, D. F. J. Catal. 1983, 82, 418.
Hisanaga, T.; Harada, K.; Tanaka, K. J. Photochem. Photobiol. A 1990, 54, 113.
Kormann, C.; Bahnemann, D.; Hoffmann, M. R. Environ. Sci. Technol. 1991, 25, 494.
Sabin, F.; Tirlúk, T.; Vogler, A. J. Photochem. Photobiol. A 1992, 63, 99.
Hingendorff, M.; Bahnemann, D. W. J. Adv. Oxid. Technol. 1996, 1, 35.
Choi, W.; Hoffmann, M. R. Environ. Sci. Technol. 1995, 29, 1646.
Choi, W.; Hoffmann, M. R. J. Phys. Chem. 1996, 100, 2161.
Minero, C.; Catozzo, F.; Pelizzetti, E. Langmuir 1992, 8, 481.
Lawless, D.; Serpone, N.; Meisel, D. J. Phys. Chem. 1991, 95, 5166.
Morrison, S. R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Plenum: New York, 1980; p 163.
Bahnemann, D. W.; Cunnigham, J.; Fox, M. A.; Pelizzetti, E.; Pichat, P.; Serpone, N. In Aquatic and Surface Chemistry; Helz, G. R.; Zepp, R. G.; Crosby, D. G., Eds.; Lewis: Boca Raton, 1994; 261.
Hoffmann, M. R.; Martin, S. T.; Choi, W.; Bahnemann, D. W. Chem. Rev 1995, 95, 69.
Pelizzetti, E.; Minero, C.; Maurino, V.; Sclafani, A.; Hidaka, H.; Serpone, N. Environ. Sci. Technol. 1989, 23, 1380.
Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Chemosphere 1993, 26, 2103.
Strukul, G. Catalytic Oxydations with Hydrogen Peroxyde as Oxidant; Kluwer Academic: Boston, 1992; p 127.
Buxton, G. V.; Greestock, C. L.; Helmann, W. P.; Ross, A. B. J. Phys. Chem. Ref. Data 1988, 17, 513.
Walling, C. H. Acc. Chem. Res. 1975, 8, 125–131.
Walling, C.; Cleary, M. Int. J. Chem. Kinet. 1977, 9, 595–601.
Markham, M. C.; Laidler, J. K. J. Phys. Chem. 1953, 57, 363.
Osawa, Y. O.; Gratzel, M. J. Chem. Soc. Faraday Trans. 1 1988, 84, 197.
Muraki, H.; Saji, T.; Fujihira, M.; Aoyagui, S. J. Electroanal. Chem. 1984, 169, 319.
Bielski, B. H. J.; Cabelli, D. E.; Arudi, R. L.; Ross, A. B. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100.
Frimer, A. A.. In Oxygen Radicals in Biology and Medicine; Simic, M. G.; et al, Eds.; Plenum Press: New York, 1988; 29–38.
Lilie, V. J.; Beck, G.; Henglein, A. Ber. Bunsen-ges Phys. Chem. 1971, 75, 458.
Micic, O.; Zhang, Y.; Cromeck, K. R.; Trifunac, A. D.; Thurnauer, M. C. J. Phys. Chem. 1993, 97, 13284.
Von Sonntag, C.. In Oxygen Radicals in Biology and Medicine; Simic, M. G.; et al, Eds.; Plenum Press: New York, 1988; 47–54.
Kunai, A.; Hata, S.; Ita, S.; Sasaki, K. J. Am. Chem. Soc. 1986, 108, 6012–6016.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Calza, P., Pelizzetti, E., Brussino, M. et al. Ion trap tandem mass spectrometry study of dexamethasone transformation products on light activated TiO2 surface. J Am Soc Mass Spectrom 12, 1286–1295 (2001). https://doi.org/10.1016/S1044-0305(01)00319-1
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(01)00319-1