Abstract
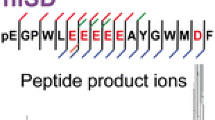
Mass spectrometry of charged derivatives of peptides has been a growing area of interest in the past decade. Fragmentation of charged derivatives of peptides is believed to be different from than that of protonated peptides when analyzed by collisionally activated dissociation-tandem mass spectrometry (CAD-MS/MS). The charged derivatives fragment by charge-remote fragmentation mechanisms, which are usually classified as high-energy (HE)-CAD processes. Our objective in the present study is to investigate the mechanism of fragmentation of charged derivatives of peptides when analyzed by matrix-assisted laser desorption/ionization-post-source decay-mass spectrometry (MALDI-PSD-MS) and electrospray ionization (ESI)-CAD-MS/MS (ion trap), which involve low-energy processes. Three major types of hydrogens (α, β, and amide) are available for migration during the formation of the *a n ions (the predominant ion series produced from these charged derivatives). To pinpoint which of the three hydrogens is involved in the formation of the *a n ions, deuterium-labeled peptide derivatives with labels at specific sites were synthesized and analyzed by MALDI-PSD-MS and ESI-CAD-MS/MS. Our results suggest that the amide hydrogen of the residue at which the cleavage occurs shifts during the formation of *a n; this observation serves as evidence for the mechanism proposed earlier by Liao et al. for fragmentation of such charged derivatives. The results also help elucidate the structure of the *a n ions, *b n ions, and others formed during cleavage at the proline residue, as well as the ions formed during loss of the C-terminal residue from these charged derivatives.
Similar content being viewed by others
References
Roepstorff, P.; Fohlman, J. Biomed. Mass Spectrom. 1984, 11, 601.
Johnson, R. S.; Martin, S. A.; Biemann, K.; Stults, J. T.; Watson, J. T. Anal. Chem. 1987, 59, 2621–2625.
Dongre, A. R.; Jones, J. L.; Somoygi, A.; Wysocki, V. H. J. Am. Chem. Soc. 1996, 118, 8365–8374.
Johnson, R. S.; Krylov, D.; Walsh, K. A. J. Mass Spectrom. 1995, 30, 386–387.
Tang, X.-J.; Thibault, P.; Boyd, R. K. Anal. Chem. 1993, 65, 2824–2834.
Jones, J. L.; Dongre, A. R.; Somogyi, V. H.; Wysocki, V. H. J. Am. Chem. Soc. 1994, 116, 8368–8369.
Hunt, D. F.; Yates, J. R.; Shabanowitz, J.; Winston, S.; Hauer, C. R. Proc. Natl. Acad. USA 1986, 83, 6233–6237.
Mueller, D. R.; Eckersley, M.; Richter, W. J. Org. Mass Spectrom. 1988, 23, 217–222.
Kenny, P.; Nomoto, K.; Orlando, R. Rapid Commun. Mass Spectrom. 1992, 6, 95–97.
Roth, K. D. W.; Huang, Z.-H.; Sadagopan, N.; Watson, J. T. Mass Spectrom. Rev. 1998, 17, 255–274.
Keough, T.; Youngquist, R. S.; Lacey, M. P. Proc. Natl. Acad. Sci. USA 1999, 96, 7131–7136.
Bauer, M. D.; Sun, Y.; Keough, T.; Lacey, M. P. Rapid Commun. Mass Spectrom. 2000, 14, 924–929.
Burlet, O.; Orkiszewski, R. S.; Ballard, K. D.; Gaskell, S. J. Rapid Commun. Mass Spectrom. 1992, 6, 658–662.
Ligon, W. V. Anal. Chem. 1986, 58, 485–487.
Huang, Z.-H.; Wu, J.; Roth, K. D. W.; Yang, Y.; Gage, D. A.; Watson, J. T. Anal. Chem. 1997, 69, 137–144.
Liao, P.-C.; Huang, Z.-H.; Allison, J. J. Am. Soc. Mass Spectrom. 1997, 8, 501–509.
Sadagopan, N.; Watson, J. T. J. Am. Soc. Mass Spectrom. 2000, 11, 107–119.
Wagner, D. S. Characterization of the triphenylphosphonium derivative of peptides by fast atom bombardment-tandem mass spectrometry, and investigations of the mechanisms of fragmentation of peptides. Ph.D. Dissertation, Michigan State University, 1992.
Barany, G.; Merrifield, R. B. The Peptide Analysis, Synthesis, Biology; Gross, E.; Meinhofer, J., Ed.; Academic: New York, 1980.
Yalcin, T.; Khouw, C.; Csizimadia, I. G.; Peterson, M. R.; J. Am. Soc. Mass Spectrom. 1995, 6, 1165–1174.
Thorne, G. C.; Ballard, K. D.; Gaskell, S. J. J. Am. Soc. Mass Spectrom. 1990, 1, 249–257.
Tang, X.; Boyd, R. K. Rapid Commun. Mass Spectrom. 1992, 6, 651–657.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadagopan, N., Watson, J.T. Mass spectrometric evidence for mechanisms of fragmentation of charge-derivatized peptides. J Am Soc Mass Spectrom 12, 399–409 (2001). https://doi.org/10.1016/S1044-0305(01)00211-2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(01)00211-2