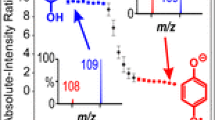
Abstract
The term “wrong-way-round ionization” has been used in studies of electrospray ionization to describe the observation of protonated or deprotonated ions when sampling strongly basic or acidic solutions (respectively) where such ions are not expected to exist in appreciable concentrations in solution. Study of the dependence of ionization of the weak base caffeine on the electrospray capillary potential reveals three distinct contributors to wrong-way-round ionization. At near-neutral pH in solutions of low ionic strength, protonation of caffeine results from the surface enrichment of electrolytically produced protons in the surface layer of the droplets from which ions are desorbed. For solutions made strongly basic with ammonia, gas-phase proton transfer from ammonium ions can create protonated caffeine. These two mechanisms have been discussed previously elsewhere. For solutions of high ionic strength at neutral or high pH, the data suggest that discharge-induced ionization is responsible for the production of protonated caffeine. This mechanism probably accounts for some of the wrong-way-round ionization reported elsewhere.
Similar content being viewed by others
References
Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. Science 1989, 246, 64–71.
Fenn, J. B. J. Am. Soc. Mass Spectrom. 1993, 4, 524–529.
Van Berkel, G. J.; Zhou, F.; Aronson, J. T. Int. J. Mass Spectrom. Ion Processes 1997, 162, 55–67.
Van Berkel, G. J. In Electrospray Ionization Mass Spectrometry; Cole, R. B., Ed.; Wiley: New York, 1997; pp 65–105.
Mansoori, B. A.; Volmer, D. A.; Boyd, R. K. Rapid Commun. Mass Spectrom. 1997, 11, 1120–1130.
Kelly, M. A.; Vestling, M. M.; Fenselau, C. C.; Smith, P. B. Org. Mass Spectrom. 1992, 27, 1143–1147.
Guevremont, R.; Siu, K. W. M.; Le Blanc, J. C. Y.; Berman, S. S. J. Am. Soc. Mass Spectrom. 1992, 3, 216–224.
Le Blanc, J. C. Y.; Wang, J.; Guevremont, R.; Siu, K. W. M. Org. Mass Spectrom. 1992, 29, 587–593.
Wang, G.; Cole, R. B. Org. Mass Spectrom. 1994, 29, 419–427.
Charbonnier, F.; Berthelot, L.; Rolando, C. Anal. Chem. 1999, 71, 1585–1591.
Loo, J. A.; Udseth, H. R.; Smith, R. D. Rapid Commun. Mass Spectrom. 1988, 2, 207–210.
Hiraoka, K.; Murata, K.; Kudaka, I. J. Mass. Spectrom. Soc. Jpn. 1995, 43, 127–138.
Konermann, L.; Collings, B. A.; Douglas, D. J. Biochemistry 1997, 36, 12296–12302.
Le Blanc, J. C. Y.; Guevremont, R.; Siu, K. W. M. Int. J. Mass Spectrom. Ion Processes 1993, 125, 145–153.
Gatlin, C. L.; Tureček, F. Anal. Chem. 1994, 66, 712–718.
Zhou, S.; Edwards, A. G.; Cook, K. D.; Van Berkel, G. J. Anal. Chem. 1999, 71, 769–776.
Tang, L.; Kebarle, P. Anal. Chem. 1993, 65, 3654–3668.
Enke, C. G. Anal. Chem. 1997, 69, 4885–4893.
Kebarle, P.; Ho, Y. In Electrospray Ionization Mass Spectrometry; Cole, R. B., Ed.; Wiley: New York, 1997; pp 1–64.
Perrin, D. D. Dissociation Constants of Organic Bases in Aqueous Solution; Butterworths: London, 1965.
Stryer, L. Biochemistry; W. H. Freeman and Company: New York, 1988.
Iribarne, J. V.; Thomson, B. A. J. Chem. Phys. 1976, 64, 2287–2294.
Thomson, B. A.; Iribarne, J. V. J. Chem. Phys. 1979, 71, 4451–4465.
CRC Handbook of Chemistry and Physics, 71st ed.; Lide, D. E., Ed.; CRC: Boca Raton, FL, 1990.
Cooks, R. G.; Patrick, J. S.; McLuckey, S. A. Mass Spectrom. Rev. 1994, 13, 287–339.
Horning, E. C.; Horning, M. G.; Carroll, D. I.; Dzidic, I.; Stillwell, R. N. Anal. Chem. 1973, 45, 936–943.
Good, A.; Durden, D. A.; Kebarle, P. J. Chem. Phys. 1970, 52, 222–229.
Sunner, J.; Nicol, G.; Kebarle, P. Anal. Chem. 1988, 60, 1300–1307.
Lias, S. G.; Bartmess, J. E.; Liebman, J. F.; Holmes, J. L.; Levin, R. D.; Mallard, W. G. J. Phys. Chem. Ref. Data 1988, 17, Suppl. 1.
Lüttgens, U.; Röllgen, F. W.; Cook, K. D. In Methods and Mechanisms for Producing Ions from Large Molecules (NATO ASI Series B, Vol. 269); Standing, K. G.; Ens, W., Eds.; Plenum: New York, 1991; pp 185–193.
Bruins, A. P. Mass Spectrom. Rev. 1991, 10, 53–77.
Harrison, A. G. Chemical Ionization Mass Spectrometry, 2nd ed.; CRC: Boca Raton, FL, 1992; pp 91–100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, S., Cook, K.D. Protonation in electrospray mass spectrometry: Wrong-way-round or right-way-round?. J. Am. Soc. Spectrom. 11, 961–966 (2000). https://doi.org/10.1016/S1044-0305(00)00174-4
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(00)00174-4