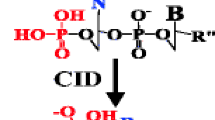
Abstract
Electrospray mass spectrometry techniques were used to characterize components of the active site in Endonuclease VIII by identifying the amino acid sequence and the binding site for a tryptic peptide derived from Endo VIII in a cross-linked DNA-peptide complex. Endo VIII, a DNA repair enzyme with both glycosylase and lyase activities, was covalently bound to a thymidine glycol-containing oligodeoxynucleotide duplex by converting a transient Schiff base formed during the course of the glycosylase activity to a stable covalent bond by chemical reduction with sodium borohydride. After tryptic digestion of the initial product, the identification of the cross-linked peptide was deduced initially from the molecular mass of the tryptic product obtained by negative ion electrospray mass analysis. Nanospray tandem mass spectrometry (MS/MS) analysis of the tryptic product corroborated the molecular mass of the peptide fragment and verified the point of attachment to the oligomer, but failed to produce sufficient fragmentation to sequence the peptide completely. Direct evidence for the amino acid sequence of the peptide was obtained after enzymatic digestion of the DNA portion of the cross-linked DNA-peptide product and analysis by negative ion nanospray MS/MS. Examination of the ions from collision induced fragmentation disclosed that this substance was the N-terminal tryptic fragment of Endo VIII cross-linked to a portion of the oligomer, and that the N-terminal proline from Endo VIII was covalently bound to the residual deoxyribose moiety at the original location of the thymine glycol in the oligomer.
Similar content being viewed by others
References
Siuzdak, G. The emergence of mass spectrometry in biochemical research. Proc. Natl. Acad. Sci. USA 1994, 91, 11290–11297.
Przybylski, M.; Kast, J.; Glocker, M. O.; Durr, E.; Bosshard, H. R.; Sprinzl, M. Mass spectrometric approaches to molecular characterization of protein-nucleic acid interactions. Toxicol. Lett. 1995, 82-83, 567–575.
Greig, M. J.; Gaus, H. J.; Griffey, R. H. Negative ionization micro electrospray mass spectrometry of oligonucleotides and their complexes. Rapid Commun. Mass Spectrom. 1996, 10, 47–50.
Potier, N.; Donald, L. J.; Chernushevich, I.; Ayed, A.; Ens, W.; Arrowsmith, C. H.; Standing, K. G.; Duckworth, H. W. Study of a noncovalent trp repressor:DNA operator complex by electrospray ionization time-of-flight mass spectrometry. Protein Sci. 1998, 7, 1388–1395.
Williams, K. R.; Konigsberg, W. H. Identification of amino acid residues at interface of protein-nucleic acid complexes by photochemical cross-linking. In Methods of Enzymology; Sauer, R. T., Ed., Academic: New York, 1991; Vol. 208, pp 516–539.
Tchou, J.; Grollman, A. P. The catalytic mechanism of fpg protein: Evidence for a Schiff base intermediate and aminoterminus localization of the catalytic site. J. Biol. Chem. 1995, 270, 11671–11677.
Wong, D. L.; Paviovich, J. G.; Reich, N. O. Electrospray ionization mass spectrometric characterization of photo cross-linked DNA-EcoRI DNA methyltrasferase complexes. Nucleic Acids Res. 1998, 26, 645–649.
Jensen, O. N.; Barofsky, D. F.; Young, M. C.; von Hippel, P. H.; Swenson, S.; Seifried, S. E. Direct observation of UV-cross-linked protein-nucleic acid complexes by matrix-assisted laser desorption ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1993, 7, 496–501.
Bennett, S. E.; Jensen, O. N.; Barofsky, D. F.; Mosbaugh, D. W. UV-catalyzed cross-linking of Escherichia coli uracil-DNA glycosylase to DNA. J. Biol. Chem. 1994, 269, 21870–21879.
Jensen, O. N.; Barofsky, D. F.; Young, M. C.; von Hippel, P. H.; Swenson, S.; Seifried, S. E. Direct observation of UV-crosslinked protein-nucleic acid complexes by matrix-assisted laser desorption ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1993, 7, 496–501.
Zharkov, D. O.; Rieger, R. A.; Iden, C. R.; Grollman, A. P. NH2-terminal proline acts as a nucleophile in the glycosylase/AP-lyase reaction catalyzed by Escherichia coli formamidopyridine-DNA glycosylase (fpg) protein. J. Biol. Chem. 1997, 272, 5335–5341.
Shneier, A.; Kleanthous, C.; Deka, R.; Coggins, J. R.; Abell, C. Observation of an imine intermediate on dehydroquinase by electrospray mass spectrometry. J. Am. Chem. Soc. 1991, 113, 9416–9418.
Melamede, R. J.; Hatahet, Z.; Kow, Y. W.; Ide, H.; Wallace, S. S. Isolation and characterization of endonuclease VIII from Escherichia coli. Biochemistry 1994, 33, 1255–1264.
Jiang, D.; Hatahet, Z.; Melamede, R. J.; Kow, Y. W.; Wallace, S. S. Characterization of Escherichia coli Endonuclease VIII. J. Biol. Chem. 1997, 272, 32230–32239.
Chang, C-H.; Beer, M.; Marzilli, L. G. Osmium-labeled polynucleotides. The reaction of osmium tetroxide with deoxyribonucleic acid and synthetic polynucleotides in the presence of tertiary nitrogen donor ligands. Biochemistry 1977, 16, 33–38.
Paleček, E. Probing DNA structure with osmium tetroxide complexes in vitro. In Methods in Enzymology, Lilley, D. M. J.; Dahlberg, J. E., Eds., Academic: New York, 1992; Vol. 212, pp 139–155.
Dodson, M. L.; Michaels, M. L.; Lloyd, R. S. Unified catalytic mechanism for DNA gylcosylases. J. Biol. Chem. 1994, 269, 32709–32712.
Reddy, D. M.; Rieger, R. A.; Torres, M. C.; Iden, C.R. Analysis of synthetic oligodeoxynucleotides containing modified components by electrospray ionization mass spectrometry. Anal. Chem. 1994, 220, 200–207.
Sambrook, J.; Fritsch, E. F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, 1989.
McLuckey, S. A.; Van Berkel, G. J.; Glish, G. L. Tandem mass spectrometry of small, multiply charged oligonucleotides. J. Am. Soc. Mass Spectrom. 1992, 3, 60–70.
Ni, J.; Pomerantz, S. C.; Rozenski, J.; Zhang, Y.; McCloskey, J. A. Interpretation of oligonucleotide mass spectra for determination of sequence using electrospray ionization and tandem mass spectrometry. Anal. Chem. 1996, 68, 1989–1999.
Roepstorff, P.; Fohlman, J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. J. Biomed. Mass Spectrom. 1984, 11, 601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rieger, R.A., McTigue, M.M., Kycia, J.H. et al. Characterization of a cross-linked DNA-Endonuclease VIII repair complex by electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 11, 505–515 (2000). https://doi.org/10.1016/S1044-0305(00)00117-3
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(00)00117-3