Abstract
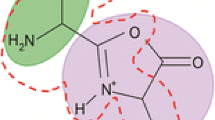
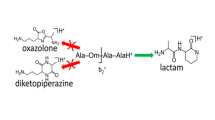
In a number of cases the b2 ion observed in peptide mass spectra fragments directly to the a1 ion. The present study examines the scope of this reaction and provides evidence as to the structure(s) of the b2 ions undergoing fragmentation to the a1 ion. The b2 ion H-Ala-Gly+ fragments, in part, to the a1 ion, whereas the isomeric b2 ion H-Gly-Ala+ does not fragment to the a1 ion. Ab initio calculations of ion energies show that this different behavior can be rationalized in terms of protonated oxazolone structures for the b2 ions provided one assumes a reverse activation energy of ∼1 eV for the reaction b2 → a2; such a reverse activation energy is consistent with experimental kinetic energy release measurements. Experimentally, the H-Aib-Ala+ b2 ion, which must have a protonated oxazolone structure, fragments extensively to the a1 ion. We conclude that the proposal by Eckart et al. (J. Am. Soc. Mass Spectrom. 1998, 9, 1002) that the b2 ions which undergo fragmentation to a1 ions have an immonium ion structure is not necessary to rationalize the results, but that the fragmentation does occur from a protonated oxazolone structure. It is shown that the b2 → a1 reaction occurs extensively when the C-terminus residue in the b2 ion is Gly and with less facility when the C-terminus residue is Ala. When the C-terminus residue is Val or larger, the b2 → a1 reaction cannot compete with the b2 → a2 fragmentation reaction. Some preliminary results on the fragmentation of a2 ions are reported.
Similar content being viewed by others
References
Tandem Mass Spectrometry; McLafferty, F. W., Ed.; Wiley: New York, 1983.
Busch, K. L.; Glish, G. L.; McLuckey, S. A. Mass Spectrometry/ Mass Spectrometry: Techniques and Applications of Tandem Mass Spectrometry; VCH: New York, 1988.
Roepstorff, P.; Fohlman, J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed. Mass Spectrom. 1984, 11, 601.
Hunt, D. F.; Yates, J. R., III; Shabanowitz, J.; Winston, S.; Hauer, C. R. Protein sequencing by tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 1986, 83, 6233.
Biemann, K.; Martin, S. Mass spectrometric determination of the amino acid sequence of peptides and proteins. Mass Spectrom. Rev. 1987, 6, 1.
Biemann, K. Contribution of mass spectrometry to peptide and protein structure. Biomed. Environ. Mass Spectrom. 1988, 16, 99.
Biemann, K. Sequencing of peptides by tandem mass spectrometry and high-energy collision-induced dissociation. Methods Enzymol. 1990, 193, 455.
Biemann, K. Primary studies of peptides and proteins. In Biological Mass Spectrometry: Present and Future; Matsuo, T.; Caprioli, R. M.; Gross, M. L.; Seyama, T., Eds.; Wiley: New York, 1993; p 275.
McCormack, A. L.; Somogyi, Á.; Dongré, A. R.; Wysocki, V. H. Fragmentation of protonated peptides: surface-induced dissociation in conjunction with a quantum mechanical approach. Anal. Chem. 1993, 65, 2859.
Somogyi, Á.; Wysocki, V. H.; Mayer, I. The effect of protonation site on bond strengths in simple peptides: application of ab initio and modified neglect of differential overlap bond order and modified neglect of differential overlap energy partitioning. J. Am. Soc. Mass Spectrom. 1994, 5, 704.
Vékey, K.; Gömöry, A. Theoretical modelling of mass spectrometric behaviour of peptides; singly and doubly protonated tetraglycine. Rapid Commun. Mass Spectrom. 1996, 10, 1485.
Wu, J.; Lebrilla, C. B. Gas-phase basicities and sites of protonation of glycine oligomers (GLY n ; n = 1–5). J. Am. Chem. Soc. 1993, 115, 3270.
Zhang, K.; Cassady, C. J.; Chung-Phillips, A. Ab initio studies of neutral and protonated triglycines: comparison of calculated and experimental gas-phase basicity. J. Am. Chem. Soc. 1994, 116, 11512.
Mueller, D. R.; Eckersley, M.; Richter, W. Hydrogen transfer reactions in the formation of “Y + 2” sequence ions from protonated peptides. Org. Mass Spectrom. 1988, 23, 217.
Johnson, R. S.; Krylov, D.; Walsh, K. A. Proton mobility within electrosprayed peptide ions. J. Mass Spectrom. 1995, 30, 386.
Harrison, A. G.; Yalcin, T. Proton mobility in protonated amino acids and peptides. Int. J. Mass Spectrom. Ion Processes 1997, 165/166, 339.
Dongré, A. R.; Jones, J. L.; Somogyi, Á.; Wysocki, V. H. Influence of peptide composition, gas-phase basicity and chemical modification on fragmentation efficiency: evidence for the mobile proton model. J. Am. Chem. Soc. 1996, 118, 8365.
Cordero, M. M.; Houser, J. J.; Wesdemiotis, C. The neutral products formed during backbone fragmentation of protonated peptides in tandem mass spectrometry. Anal. Chem. 1993, 65, 1594.
Papayannopoulos, A. The interpretation of collision-induced dissociation tandem mass spectra of peptides. Mass Spectrom. Rev. 1995, 14, 49.
Tsang, C. W.; Harrison, A. G. Chemical ionization of amino acids. J. Am. Chem. Soc. 1976, 98, 1301.
Bouchoux, G.; Bourcier, S.; Hoppilliard, Y.; Mauriac, C. Leucine and isoleucine in chemical ionization and plasma desorption. A comparative study. Org. Mass Spectrom. 1993, 28, 1064.
Dookeran, N. N.; Yalcin, T.; Harrison, A. G. Fragmentation reactions of protonated α-amino acids. J. Mass Spectrom. 1996, 31, 500.
van Dongen, W. D.; Heerma, W.; Havenkamp, J.; DeKoster, C. G. The B1-fragment ion from protonated glycine is an electrostatically-bound ion/molecule complex of CH2=NH +2 and CO. Rapid Commun. Mass Spectrom. 1996, 10, 1237.
Yalcin, T.; Khouw, C.; Csizmadia, I. G.; Peterson, M. R.; Harrison, A. G. Why are B ions stable species in peptide mass spectra. J. Am. Soc. Mass Spectrom. 1995, 6, 1165.
Arnott, D.; Kottmeir, D.; Yates, N.; Shabanowitz, J.; Hunt, D. F. Fragmentation of multiply protonated peptides under low energy conditions. Proceedings of the 42nd ASMS Conference on Mass Spectrometry; Chicago, June, 1994; p 470.
Yalcin, T.; Csizmadia, I. G.; Peterson, M. R.; Harrison, A. G. The structure and fragmentation of B n (n ≥ 3) ions in peptide spectra. J. Am. Soc. Mass Spectrom. 1996, 7, 293.
Nold, M. J.; Wesdemiotis, C.; Yalcin, T.; Harrison, A. G. Amide bond dissociation in protonated peptides. Structures of the N-terminal ionic and neutral fragments. Int. J. Mass Spectrom. Ion Processes 1997, 164, 137.
Reid, G. E.; Simpson, R. J.; O’Hair, R. A. J. A mass spectrometric and ab initio study of the pathways for the dehydration of simple glycine and cysteine-containing peptide [M + H]+ ions. J. Am. Soc. Mass Spectrom. 1998, 9, 945.
Paisz, B.; Lendvay, G.; Vékey, K.; Suhai, S. Formation of b2=NH +2 ions from protonated peptides: an ab initio study. Rapid Commun. Mass Spectrom. 1999, 13, 525.
Summerfield, S. G.; Bolgar, M. S.; Gaskell, S. J. Promotion and stabilization of b1 ions in peptide phenylthiocarbamoyl derivatives. Analogies with condensed-phase chemistry. J. Mass Spectrom. 1997, 32, 225.
Yalcin, T.; Gabryelski, W.; Li, L. Dissociation of protonated phenylthiohydantoin amino acids and phenylthiocarbamoyl dipeptides. J. Mass Spectrom. 1998, 33, 543.
Pfeifer, T.; Schierhorn, A.; Friedemann, R.; Jakob, M.; Frank, R.; Schutkowski, M.; Fischer, G. Specific fragmentation of thioxo peptides facilitates the assignment of the thioxylated amino acid. J. Mass Spectrom. 1997, 32, 1064.
Vaisar, T.; Urban, J. Gas-phase fragmentation of mono-N-methylated peptides. Analogy with solution-phase acid-catalyzed hydrolysis. J. Mass Spectrom. 1998, 33, 505.
Lee, V. W.-M.; Li, H.; Lau, T.-C.; Siu, K. W. M. Structures of b and a product ions from the fragmentation of argentinated peptides. J. Am. Chem. Soc. 1998, 120, 7302.
Eckart, K.; Holthausen, M. C.; Koch, W.; Spiess, J. Mass spectrometric and quantum mechanical analysis of gas-phase formation, structure and decomposition of various b2 ions and their specifically deuterated analogs. J. Am. Soc. Mass Spectrom. 1998, 9, 1002.
Ambihapathy, K.; Yalcin, T.; Leung, H.-W.; Harrison, A. G. Pathways to immonium ions in the fragmentation of protonated peptides. J. Mass Spectrom. 1997, 32, 209.
McLuckey, S. A.; Glish, G. L.; Cooks, R. G. Kinetic energy effects in mass spectrometry/mass spectrometry using a sector/quadrupole tandem instrument. Int. J. Mass Spectrom. Ion Phys. 1981, 39, 219.
Fetterolf, D. D.; Yost, R. A. Energy-resolved collision-induced dissociation in tandem mass spectrometry. Int. J. Mass Spectrom. Ion Phys. 1982, 44, 37.
McLuckey, S. A.; Cooks, R. G. Angle- and energy-resolved fragmentation spectra from tandem mass spectrometry. In Tandem Mass Spectrometry; McLafferty, F. W., Ed.; Wiley: New York, 1983; p 303.
Bruins, A. P.; Jennings, K. R.; Stradling, R. S.; Evans, S. Observation of metastable transitions in a double-focussing mass spectrometer using a linked scan of the electric sector and the magnetic sector fields. Int. J. Mass Spectrom. Ion Phys. 1978, 26, 395.
Jennings, K. R.; Dolnikowski, G. G. Mass analyzers. Methods Enzymol. 1990, 193, 37.
Bruins, A. P. ESI source design and dynamic range considerations. In Electrospray Mass Spectrometry: Fundamentals, Instrumentation and Applications; Cole, R. B., Ed.; Wiley: New York, 1997; Chap 3.
Donò, A.; Paradisi, C.; Scorrano, G. Abatement of volatile organic compounds by corona discharge. A study of the reactivity of trichloroethylene under atmospheric pressure ionization conditions. Rapid Commun. Mass Spectrom. 1997, 11, 1687.
Collette, C.; DePauw, E. Calibration of the internal energy distribution of ions produced by electrospray. Rapid Commun. Mass Spectrom. 1998, 12, 165.
Collette, C.; Drahos, L.; DePauw, E.; Vékey, K. Comparison of the internal energy distributions of ions produced by different electrospray sources. Rapid Commun. Mass Spectrom. 1998, 12, 1673.
Harrison, A. G. Energy-resolved mass spectrometry. A comparison of quadrupole cell and cone-voltage collision-induced dissociation. Rapid Commun. Mass Spectrom. 1999, 13, 1663.
Harrison, A. G. Fragmentation reactions of alkylphenyl ammonium ions. J. Mass Spectrom. 1999, 34, 1253.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Gill, P. M. W.; Johnson, B. G.; Robb, M. A.; Cheeseman, J. R.; Keith, T. A.; Petersson, G. A.; Montgomery, J. A.; Raghavachari, K.; Al-Laham, M. A.; Zakrzewski, G. G.; Ortiz, J. V.; Foresman, J. B.; Cioslowski, J.; Stefanov, B. B.; Nanayakkara, A.; Challacombe, M.; Peng, C. Y.; Ayala, P. Y.; Chen, W.; Wong, M. W.; Andres, J. L.; Replogle, E. S.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Binkley, J. S.; DeFrees, D. J.; Baker, J.; Stewart, J. P.; Head-Gordon, M.; Gonzalez, C.; Pople, J. A. GAUSSIAN 94, Revision A.1, Gaussian Inc., Pittsburgh, PA, 1995.
Derrick, P. J.; Donchi, K. F. Mass spectrometry. In Comprehensive Chemical Kinetics, Vol 24; Bamford, C. H.; Tipper, C. F. H., Eds.; Elsevier Scientific: Amsterdam, 1983, p 53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrison, A.G., Csizmadia, I.G. & Tang, TH. Structure and fragmentation of b2 ions in peptide mass spectra. J Am Soc Mass Spectrom 11, 427–436 (2000). https://doi.org/10.1016/S1044-0305(00)00104-5
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(00)00104-5