Abstract
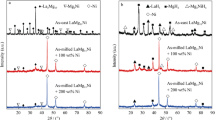
Nanocrystalline and amorphous LaMg12-type alloy-Ni composites with a nominal composition of LaMg11Ni+x wt. % Ni (x = 100, 200) were synthesized via ball milling. The influences of ball milling duration and Ni adding amount x on the gaseous and electrochemical hydrogen storage dynamics of the alloys were systematically studied. Gaseous hydrogen storage performances were studied by a differential scanning calorimeter and a Sievert apparatus. The dehydrogenation activation energy of the alloy hydrides was evaluated by Kissinger method. The electrochemical hydrogen storage dynamics of the alloys was investigated by an automatic galvanostatic system. The H atom diffusion and apparent activation enthalpy of the alloys were calculated. The results demonstrate that a variation in Ni content remarkably enhances the gaseous and electrochemical hydrogen storage dynamics performance of the alloys. The gaseous hydriding rate and high-rate discharge (HRD) ability of the alloys exhibit maximum values with varying milling duration. However, the dehydriding kinetics of the alloys is always accelerated by prolonging milling duration. Specifically, rising milling time from 5 to 60 h makes the hydrogen desorption ratio (a ratio of the dehydrogenation amount in 20 min to the saturated hydrogenation amount) increase from 57% to 66% for x = 100 alloy and from 57% to 70% for x = 200. Moreover, the improvement of gaseous hydrogen storage kinetics is attributed to the descending of dehydrogenation activation energy caused by the prolonging of milling duration and growing of Ni content.
Similar content being viewed by others
References
D. Mori, K. Hirose, Int. J. Hydrogen Energy 34 (2009) 4569–4574.
H. Fayaz, R. Saidur, N. Razali, F. S. Anuar, A. R. Saleman, M. R. Islam, Renew. Sust. Energ. Rev. 16 (2012) 5511–5528.
Y. H. Zhang, Z. M. Yuan, W. G. Bu, F. Hu, Acta Metall. Sin. (Engl. Lett.) 29 (2016) 577–586.
T. Yang, Z. M. Yuan, W. G. Bu, Z. C. Jia, Y. Qi, Y. H. Zhang, Int. J. Hydrogen Energy 41 (2016) 2689–2699.
T. Umegaki, J. M. Yan, X. B. Zhang, S. Hiroshi, K. Nobuhiro, Q. Xu, Int. J. Hydrogen Energy 34 (2009) 2303–2311.
Y. H. Zhang, Z. C. Jia, Z. M. Yuan, T. Yang, J. Iron Steel Res. Int. 22 (2015) 757–770.
Y. Wang, S. Z. Qiao, X. Wang, Int. J. Hydrogen Energy 33 (2008) 5066–5072.
Z. M. Yuan, T. Yang, W. G. Bu, H. W. Shang, Y. H. Zhang, Int. J. Hydrogen Energy 41 (2016) 5994–6003.
L. P. Jain, C. Lal, A. Jain, Int. J. Hydrogen Energy 35 (2010) No. 10, 5133–5144.
A. Zaluska, L. Zaluski, J. Strom-Olsen, J. Alloys Comp. 288 (1999) 217–225.
F. C. Gennari, M. R. Esquivei, J. Alloys Comp. 459 (2008) 425–432.
T. Spassov, U. Lyubenova, U. Köster, M. D. Barò, Mater. Sci. Eng. A 375–377 (2004) 794–799.
A. P. Andrey, V. D. Roman, P. M. Jan, K. S. Jan, P. T. Boris, A. Y. Volodymyr, Int. J. Hydrogen Energy 37 (2012) 3548–3557.
Y. Wang, X. Wang, C. M. Li, Int. J. Hydrogen Energy 35 (2010) 3550–3554.
Q. A. Zhang, C. J. Jiang, D. D. Liu, Int. J. Hydrogen Energy 37 (2012) 10709–10714.
M. Abdellaoui, S. Mokbli, F. Cuevas, M. Latroche, A. Percheron-Gue’gan, H. Zarrouk, J. Alloys Comp. 356–357 (2003) 557–561.
L. Z. Ouyang, J. M. Huang, C. J. Fang, Q. A. Zhang, D. L. Sun, M. Zhu, Int. J. Hydrogen Energy 37 (2012) 12358–12364.
A. Mustafa, K. Fatma, K. Nilüfer, Int. J. Hydrogen Energy 37 (2012) 299–308.
T. Sadhasivam, M. Sterlin Leo Hudson, K. P. Sunita, B. Ashish, K. S. Milind, K. Gurunathan, O. N. Srivastava, Int. J. Hydrogen Energy 38 (2013) 7353–7362.
H. E. Kissinger, Anal. Chem. 29 (1957) 1702–1706.
Y. Wu, W. Han, S. X. Zhou, M. V. Lototsky, J. K. Solberg, V. A. Yartys, J. Alloys Comp. 466 (2008) 176–181.
D. H. Xie, P. Li, C. X. Zeng, J. W. Sun, X. H. Qu, J. Alloys Comp. 478 (2009) 96–102.
M. Y. Song, C. D. Yim, S. N. Kwon, J. S. Bae, S. H. Hong, Int. J. Hydrogen Energy 33 (2008) 87–92.
M. Anik, J. Alloys Comp. 491 (2010) 565–570.
X. Y. Zhao, Y. Ding, L. Q. Ma, L. Y. Wang, M. Yang, X. D. Shen, Int. J. Hydrogen Energy 33 (2008) 6727–6733.
G. Zheng, B. N. Popov, R. E. White, J. Electrochem. Soc. 142 (1995) 2695–2698.
N. Kuriyama, T. Sakai, H. Miyamura, I. Uehara, H. Ishikawa, T. Iwasaki, J. Alloys Comp. 202 (1993) 183–197.
Y. H. Zhang, P. J. Zhang, Z. M. Yuan, T. Yang, Y. Qi, D. L. Zhao, J. Rare Earths 33 (2015) 874–883.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, Dc., Sun, H., Hou, Zh. et al. Highly ameliorated gaseous and electrochemical hydrogen storage dynamics of nanocrystalline and amorphous LaMg12-type alloys prepared by mechanical milling. J. Iron Steel Res. Int. 24, 50–58 (2017). https://doi.org/10.1016/S1006-706X(17)30008-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1006-706X(17)30008-0