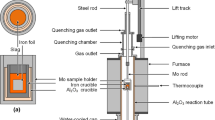
Abstract
The enrichment behavior of phosphorus in CaO-SiO2-FeOx-P2O3 slag was studied by making an investigation on the distribution of phosphorous content in the CaO-SiO2-FeOx-P2 O5 molten slag. The research results showed that the 2CaO · SiO2 solid particles existing in molten slag were the condensation sites for the phosphorus enrichment. The enrichment process of phosphorus in the molten slag can be recognized as three substeps: mass transfer of phosphorus from bulk slag to the surface of 2CaO · SiO2 particle, superficial solid solution reaction of phosphorus around the 2CaO · SiO2 particle, and diffusion of phosphorus through the product layer of 2CaO · SiO2-3CaO · P2O5 solid solution to the inner of 2CaO · SiO2 particle. Moreover, higher temperature is favorable to the phosphorous enrichment from molten bulk slag to the 2CaO · SiO2 particles.
Similar content being viewed by others
References
Dippenaar R. Industrial Uses of Slag—the Use and Re-Use of Iron and Steelmaking Slags [J]. Ironmaking and Steelmaking, 2005, 32(1): 35.
Morita K, Guo M X, Oka N, et al. Resurrection of the Iron and Phosphorus Resource in Steel-Making Slag [J]. J Mater Cycles Waste Manag, 2002, 4(2): 93.
Boom R, Riaz S, Mills K C. Slags and Fluxes Entering the New Millennium, an Analysis of Recent Trends in Research and Development [J]. Ironmaking and Steelmaking, 2005, 32(1): 21.
Li H J, Suito H, Tokuda M. Thermodynamic Analysis of Slag Recycling Using a Slag Regenerator [J]. ISIJ International, 1995, 35(9): 1079.
LI Liao-sha, YU Xue-feng, WU Xing-rong, et al. Distribution of Phosphorus in Converter Steel Slags Stabilized in Different Ways [J]. Metal Mine, 2006, (10): 78.
Agrawal D K, Maslowski A R, Adair J H. Evolution of the Formation of Inorganic Polymers in the CaO-SiO2-P2O5 System Using Metal Alkoxides [J]. J Am Ceram Soc, 1990, 73(2): 430.
Inoue R, Suito H. Phosphorous Partition Between 2CaO · SiO2 Particles and 2CaO-SiO2-Fe2O Slags [J]. ISIJ International, 2006, 46(2): 174.
Inoue R, Suito H. Mechanism of Dephosphorization With CaO- SiO2-FexO Slags Containing Mesoscopic Scale 2CaO · SoO2 Particles [J]. ISIJ International, 2006, 46(2): 188.
Shimauchi K I, Kitamura S Y, Shibata H. Distribution of P2O5 Between Solid Dicalcium Silicate and Liquid Phases in CaO-SiO2-Fe2O3 System [J]. ISIJ International, 2009, 49(4): 505.
Yang X, Matsuura H, Tsukihashi F. Condensation of P2O5 at the Interface between 2CaO · SoO2 and CaO-SiO2FeOx-P2O5 Slag [J]. ISIJ International, 2009, 49(9): 1298.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation Item: Item Sponsored by National Natural Science Foundation of China/Joint Fund for Iron and Steel Research (50874130); National Natural Science Foundation of China (50974034)
Rights and permissions
About this article
Cite this article
Wang, N., Liang, Zg., Chen, M. et al. Phosphorous Enrichment in Molten Adjusted Converter Slag: Part II Enrichment Behavior of Phosphorus in CaO-SiO2-FeOx-P2O5 Molten Slag. J. Iron Steel Res. Int. 18, 22–26 (2011). https://doi.org/10.1016/S1006-706X(12)60004-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1006-706X(12)60004-1