Abstract
Extremely preterm infants are prone to hyperglycemia which is associated with increased mortality and morbidity. Insulin sensitivity is variable in extreme prematurity, and its monitoring, prevention, and treatment are a significant challenge in the NICU. Frequent changes in fluid composition and volumes, as well as large growth and adaptational nutrient requirements are limited by difficult vascular access and blood sampling and risk of drug incompatibilities. Insulin treatment requires specific access and significantly increases fluid intake and sampling. Clinicians, therefore, often compromise by reducing glucose intake and accepting higher glycemia. We report a case series of 11 extremely low birth weight (ELBW) preterms, born between 23 5/7 and 27 6/7 weeks of gestation, treated for transient hyperglycemia during the first 2 weeks of life by continuous subcutaneous insulin infusion (CSII). Insulin concentration was 10 IU/ml, administered via 13 mm Accu-Chek® Tenderlink catheters and a commercial insulin pump (Accu-Chek® Combo, Roche Diabetes Care). Insulin treatment was initiated when glycemia was > 252 mg/dL (14 mmol/l) in two consecutive blood glucose determinations, except for one case when glycemia was 234 mg/dL (13 mmol/l), despite a previous decrease in glucose infusion rate. The starting dose for the CSII was between 0.01 and 0.08 IU/kg/h. The average duration of the CSII was 5 days (1–16 days). CSII in extreme preterm neonates with hyperglycemia was clinically feasible and practical by sparing IV lines and volume and appeared as more rapidly effective than continuous IV administration. No adverse events like hypoglycemia or skin infection were recorded.
Similar content being viewed by others

Introduction
Hyperglycemia in newborns is usually defined by plasma glucose concentrations ≥ 150 mg/dL (8.3 mmol/L) [1]. Studies examining consequences of hyperglycemia or treatment thresholds have used varying and higher glucose thresholds: Zamir et al. set levels ≥ 180 mg/dL (10 mmol/L) when evaluating consequences, and Lemelman et al. recommend treatment with insulin if glucose levels are ≥ 250 mg/dL (13.8 mmol/L) [2, 3].
This condition occurs frequently in extremely low birth weight (ELBW) neonates, especially during the first days after birth. The risk is inversely related to gestational age and birth weight and increases with the severity of accompanying diseases: acute intracranial bleeding, necrotizing enterocolitis, sepsis, and surgery are stressing events that frequently trigger hyperglycemia. Hyperglycemia may also be induced by medications, such as caffeine, steroids, or vasoactive drugs [4].
Neonatal hyperglycemia has been associated with adverse clinical outcomes in the literature, such as increased mortality [5,6,7], grade 3 and 4 intraventricular hemorrhage (IVH) [5], late onset bacterial [6] and fungal infection [8, 9], retinopathy of prematurity [10,11,12], necrotizing enterocolitis (NEC) [6], bronchopulmonary dysplasia [5], and prolonged length of stay in hospital [5].
This association of hyperglycemia with adverse outcomes has led to frequent treatment of the condition with insulin at different thresholds, although its benefits remain a matter of debate in terms of improvement of survival and decrease in morbidity [13, 14]. Treatment thresholds vary in clinical practice between centers and target prevention of metabolic complications such as increase in blood osmolality or dehydration that occur from osmotic diuresis.
In preterm neonates, insulin is usually administered intravenously (IV). The continuous infusion rate may vary from 0.1 to several ml/h, leading to large fluid volumes administration for ELBW, and the treatment requires in general a dedicated venous access. An unpredictable delay in onset of the insulin action due to adsorption of insulin to the polyvinyl or polyethylene tubing of the central lines, often of many hours, further complicate the treatment. The risk of inadvertent hypoglycemia during this initiation of treatment, until steady state, therefore, necessitates frequent invasive blood glucose measurements.
Continuous subcutaneous insulin infusion (CSII) may present several advantages over IV administration: a higher insulin concentration leads to lower fluid volumes, less adsorption of insulin to the tubes and catheters, and the access is arguably less invasive and at lower risk of infections but clearly eases access in ELBW where venous lines are notoriously complicated.
Most of experiences available in the literature report the use of CSII for neonatal diabetes in term newborns [15,16,17,18] and only few case reports applied this practice to treat transient neonatal hyperglycemia in ELBW [19, 20]. The first experience of successful CSII treatment in a ELBW neonate born at 28 weeks postmenstrual age with transient neonatal diabetes 13 days after birth was reassuring and was used as a base for starting a CSII protocol [21].
To our knowledge, we report the largest case series of CSII treatment in 11 ELBW born between 23 and 27 weeks of gestation and presenting neonatal hyperglycemia during the first 2 weeks of life.
Methods
Context
We started our CSII protocol with a high security profile setting the threshold for CSII to a high level to reduce the risk of iatrogenic hypoglycemia. We based our protocol on theoretical considerations and literature [16,17,18]. However, to account for individual variations in response, we also tailored the initial and subsequent doses based on our previous experience [21]. Several concerns had to be overcome for the use of CSII in ELBW infants. The insertion and use of a subcutaneous catheter had to be adapted to the tiny anatomic insertion sites and scarce subcutaneous tissue. The precise volume of insulin solution to flush the subcutaneous catheter after insertion and avoid spill-over with inadvertent overdosage had to be measured. The effect of subcutaneous insulin being delayed in onset and prolonged after interruption of the perfusion, we had to design a dose-adjustment protocol to avoid overdosage and subsequent hypoglycemia. Finally, the neonatal intensive care unit (NICU) staff had to be trained to use subcutaneous insulin pumps safely.
Patients
Eleven ELBW neonates, 5 girls and 6 boys, born between 23 5/7 and 27 6/7 weeks of gestation needing insulin treatment for transient hyperglycemia during the first 2 weeks of life were included. One ELBW neonate, treated successfully by CSII was not included because the parents have denied consent to the use of the medical data.
Birth weight ranged from 415 to 890 g, with two patients presenting intrauterine growth restriction (IUGR) [case 6, 415 g, below the 3rd percentile (< P3), and case 9, 690 g, between 3rd and 5th percentile (P3–5)]. All ELBW babies were on partial oral feeding (at least a priming of human milk at 10 ml/kg/day) and parenteral nutrition with an average glucose infusion rate of 6.3 mg/kg/min (range 4.5–8 mg/kg/min). Three patients had sepsis, confirmed by blood culture; eight were under hydrocortisone therapy due to chronic or acute lung disease and one had isolated intestinal perforation with complete recovery after surgery. The most important clinical characteristics of the 11 newborns and their management are summarized in Table 1.
At the beginning of our experience, three cases (cases 1, 2, and 3) were initiated on IV insulin treatment and switched to CSII after 1 to 9 days until treatment could be stopped. Another patient (case 11) received IV insulin at the discretion of the physician in charge but was switched to CSII on day 3 due to difficult venous access after peripheral venous catheter extravasation. In cases 5, 7, 8, 9, and 10 we directly administered insulin by the pump. Case 4 received CSII at day 1, with a persistent hyperglycemic state in a clinical setting of stage 4 IVH, pulmonary hemorrhage, and extreme metabolic acidosis, causing death at day 2. In one case (case 6), CSII administered from day 1 to day 8, did not allow a satisfactory glycemic control, but neither did the last 2 days of IV administration: the patient had severe IUGR < P3 and NEC when CSII was initiated, and deceased at day 10 due to septic shock.
Insulin treatment, whether IV or SC (subcutaneous), was initiated when glycemia was > 252 mg/dL (14 mmol/L), except for case 11, who was started on IV insulin, when glycemia was 234 mg/dL (13 mmol/l).
The starting dose for CSII was between 0.01 and 0.08 IU/kg/h. The average duration of CSII was 5 days (1–16 days).
Continuous subcutaneous insulin infusion
CSII was performed using an Accu-Chek® Combo insulin pump and 13 mm Accu-Chek® Tenderlink catheters (Roche Diabetes Care) which were inserted subcutaneously into the patients' thighs (as shown in Fig. 1). These insulin pumps were not originally designed for neonatal use, which mean that the standard insulin concentration of 100 IU/ml would have limited delivery in ELBW to a minimal rate of 0.05 IU/h. As we considered minimal rates of 0.005 IU/h to be necessary, the insulin was diluted 1/10 to reach a concentration of 10 IU/ml, thus allowing minimal perfusion rates of 0.005 IU/h Since the commercial pumps used displayed insulin dosage instead of infusion rate, our ‘non-standard’ insulin concentrations required continuous recalculation of insulin dosage. To avoid calculation errors, we used a dosage spreadsheet to convert the information displayed on the pump to the dose delivered to the patient (as shown in Fig. 2).
The insulin dose was systematically adapted to the patient’s glycemic values that were measured frequently, every 1 to 3 h, especially at therapy onset. The standard concentration of ultra-rapid insulin (Insulin aspart, 100 IU/mL, 10 mL, NovoRapid®, Novo Nordisk Pharma AG, Zürich, Switzerland) was used by the pharmacy to prepare sterile insulin glass vials (40 IU/4 mL in NaCl 0.9%). The subcutaneous catheter and its site were systematically changed by trained personnel every 3 days without any report of local inflammatory reaction. Tubing and insulin cartridges were simultaneously changed.
Glucose control
Blood glucose controls were all performed by Point-of-Care Test (POCT), either with glucometers (Accu-Chek Aviva®, 4 mcl) or an ABL blood gas analyzer (ABL800 FLEX® Radometer, 30 mcl). Following reference values were used: hypoglycemia was defined as blood glucose level < 47 mg/dL (2.6 mmol/L) [22] and hyperglycemia was defined as blood glucose level > 150 mg/dL (8.3 mmol/L) [1].
A standard operation procedure (SOP) was used to maintain target blood glucose levels within safe boundaries, particularly to prevent hypoglycemia.
As the risks of iatrogenic hypoglycemia on insulin treatment were considered higher than those of hyperglycemia, the threshold for insulin therapy was set to a high level: treatment was started after two consecutive blood glucose values over 252 mg/dl (14 mmol/l) within 3 h after reducing glucose infusion to a minimal rate of 6 mg/kg/min.
CSII (NovoRapid®, 10 IU/mL) dosage was initiated at medical discretion (see Table 1) and after a learning curve we achieved the following recommendation:
-
For glycemia between 252 and 288 mg/dL (14–16 mmol/L): start at 0.01–0.03 IU/kg/h,
-
For glycemia > 288–360 mg/dL (> 16–20 mmol/L): start at 0.04–0.06 IU/kg/h,
-
For glycemia > 360 mg/dL (> 20 mmol/L): start at 0.07–0.1 IU/kg/h.
Blood glucose was measured every 30 min in the first hours after initiation of CSII to check treatment response and prevent hypoglycemia. After reaching a steady state, blood controls were spaced to 1, 2 or 3 h depending on treatment response and trend. In case of insulin dose changes, blood glucose was rechecked after 30 min. Treatment was stopped when glycemia was between 144 and 180 mg/dL (8–10 mmol/L).
The treatment was continuously reported on an automated Microsoft Excel® spreadsheet, with immediate calculation and visualization of insulin dosage administered (IU/kg/h) and infusion rate (ml/h), as well as blood glucose values and glucose infusion rate.
Each new laboratory value was interpreted by a neonatologist to adjust insulin infusion rate. In case of hypoglycemia, the recommendation was to stop the pump without disconnection and to treat immediately with an IV glucose bolus followed by 15 min checks until normalization.
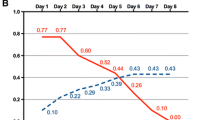
As an example, we report the trend of glycemia and insulin treatment in one of our patients (case 2) in Fig. 3. The characteristics of the patient and his treatment are summarized in Table 1.
Discussion
Medico-technical aspects
We treated 11 ELBW preterm neonates by subcutaneous insulin with good results in 9 patients. Two patients died of IVH and sepsis respectively (cases 4 and 6), without a satisfactory glycemic control by CSII, probably due the severe clinical course. Overall, the subcutaneous technique was effective and efficient, with a fast response time, allowing to spare IV lines and to reduce volume administration. We didn’t experience any adverse events: even if in 1 case we observed a rapid decrease in blood glucose, with consequent immediate interruption of the pump, none of the patients had iatrogenic hypoglycemia. No inflammatory skin reaction or infection of the subcutaneous infusion site was observed.
To implement CSII treatment, we had to overcome technical and educational challenges. Since there was no specific insulin pump available for newborns on the market, we had to use an insulin pump designed to treat patients with type 1 diabetes (Accu-Chek® Combo insulin pump), which was loaded with 1/10 diluted insulin. To avoid potential dosage errors, we had to secure this off-label use of the pump, and to this end, we developed an automated Microsoft Excel® calculation spreadsheet. This spreadsheet used the individual weight and insulin concentration of the treated neonate to calculate the effective insulin infusion rate (IU/kg/h) and flow rate (ml/h), which replaced the displayed pump infusion rate.
The introduction of the CSII technique relied on multidisciplinary collaboration between pharmacologist, pediatric diabetologists, nurses specialized in diabetes, and neonatology physicians and nurses. The pharmaceutical expertise was necessary to determine the insulin concentration and dilution, the choice of the appropriated dilution medium, and to evaluate the stability of the preparation. The advice of the pediatric diabetologists and nurses was useful for the choice of the insulin pump and perfusion material and to gain experience with the use of SC insulin and its particular dynamics. Finally, the input by nurses specialized in diabetes was necessary to implement the technical knowledge into the neonatology team, as described below.
Pharmaceutical considerations when using CSII in the neonate
Adsorption of insulin to the infusion material
One of the reasons for unpredictable delays in the onset of action during continuous IV administration of insulin in neonates is the adsorption of insulin to the infusion material. Adsorption is inversely proportional to the insulin concentration and has been observed with different material such as polyvinylchloride, polyethylene and polypropylene. Adsorption may occur in the syringe, tubing or in-line filters [23, 24]. This issue may be clinically insignificant in adults or children, but it plays a major role in the effect variability and delay observed in neonates due to the very low concentrations and flow rates of insulin used in this population [25]. The Accu-Chek® Combo pump's cartridge system and infusion set for subcutaneous use contain plastic material that may result in adsorption, similar to IV infusion systems. However, due to a higher insulin concentration, and reduced contact surface of the syringe and shorter subcutaneous infusion set, we may assume a less significant adsorption, contributing to decreased variability and delay in insulin delivery.
Choice of the insulin concentration
In the limited literature available, concentrations of either 10 IU/mL or 40 IU/mL have been used for CSII [16, 18, 26, 27]. After considering the expected doses, reasonable subcutaneous volumes, and previous publications, we concluded that the 10 IU/mL concentration was the optimal choice.
Choice of the dilution medium
The chosen insulin product (NovoRapid®,) contains two preservatives: phenol 1.5 mg/mL, and metacresol 1.72 mg/ml. Initially, we considered using the same preservatives as the insulin dilution medium. For safety reasons, we excluded these excipients due to a potential neurotoxicity in preterm neonates. We estimated the maximum exposition dose of a 1-kg patient receiving 0.1 IU/kg/h to be 2.4 IU/day, or 0.16 mg/day of phenol (the maximum recommended dose in adults is 50 mg/day, and the lethal dose is 1000 mg) and 0.14 mg/day of metacresol (considered at minimal risk for level 0.1 mg/g/day) [17, 28]. We deemed this level of risk to be inappropriate and decided to dilute insulin with unpreserved NaCl 0.9%.
Preparation of the dilution
To minimize the microbiological risk of the dilution process, we initially considered the possibility to prepare and fill the Accu-Chek® Combo pump reservoir in the pharmacy under sterile conditions. However, due to the proprietary reservoir's inability to be sealed for long-term storage after filling and its lack of tamper-proof features, we had to explore other options. The pharmacy preparation of insulin at 10 IU/mL in sterile syringes (40 IU/4 mL in NaCl 0.9%) for later transfer by the nurse into the pump reservoir unfortunately showed rapidly degradation of insulin over the time. We finally produced sterile insulin glass vials (40 IU/4 mL in NaCl 0.9%) with a stability of 3 months in the fridge (+ 2 to + 8 °C), that could be stocked in the unit and was easily transferrable to the pump reservoir for CSII.
Stability of the insulin dilution in the reservoir
Insulin aspart (NovoRapid®, NovoLog®) is chemically stable for 6 days in the Accu-Chek cartridge system [29]. Considering microbiological stability, our institutional good practice is to use continuous intravenous (IV) drug solutions for a maximum of 24 h to limit the microbiological risk of parenteral infusion. The subcutaneous administration of insulin presented risks of flow rate irregularities and inadvertent injection of an initial bolus at each solution replacement. As the administration is subcutaneous and not IV, we decided to use the insulin solution for up to 72 h before changing the pump reservoir.
Nursing aspects
To ensure the safety and reliability of insulin administration, two Clinical Nurse Specialists (CNS) in neonatology and diabetology, in collaboration with the medical team and the pharmacist, trained a small team of reference nurses in using the Accu-Chek Combo® pump for CSII.
Three challenges were identified for the efficient implementation of this complex procedure:
-
1.
The placement of the 13 mm subcutaneous catheter (Accu-Chek Tender Link Canula®) proved to be a challenge due to the length of the catheter (13 mm), which represents almost 2/3 of the thigh length of an ELBW infant. Particular care was needed to avoid cutaneous lesions and limit leg movements during the procedure.
-
2.
Changing from a standard 100 IU/mL insulin solution to a 10-IU/mL solution to increase the precision of weight-adapted insulin dosage resulted in a discrepancy between the dose displayed on the screen and the actual dose administered. This required the nurse team to recalculate the dose manually.
-
3.
Maintenance of nurse competence in the use of CSII was challenging, given the low exposure to the technique in their daily practice (5–6 cases/year).
A detailed, written step-by-step procedure was developed for all components, starting from the device preparation and reservoir filing with diluted insulin, pump programming and dosage rates according to a patient-specific dosage chart. A training was organized and delivered by the CNS on a one-on-one nurse teaching by completing the entire procedure. In addition, the CNS was called in, and performed bedside coaching during the first real-life set-up, as well as follow-up during team shifts. No incidents have been reported during this implementation phase regarding the set-up of the system, the handling of the pump or possible skin lesions.
Conclusions
Our experience with CSII by pump in extreme preterm neonates has confirmed the advantages of this technique (sparing venous catheters, reducing volume infusion, avoiding drugs interactions, efficacy and practicality) but several safety issues need to be addressed before the technique can be adopted as standard of care in NICUs. The necessary dosage dilution leads to an off-label use of the pediatric insulin pump, designed for management of diabetes in much larger children and adults. The risk of inadvertent hypoglycemia requires highly qualified personnel for the pump management, which depends on rigorous training of medical and nursing staff before and during the application of the technique.
The use of CSII was welcomed by the nursing and medical staff. Further studies on management of the pump in ELBW and adaptation of this already-existing technology in neonatal care, which is likely possible via a software update, could make treatment safer by providing the effective insulin administration directly. In this case series, we have demonstrated a safe duration of three days for the subcutaneous catheter, which we changed after this duration as a safety procedure. The relatively high treatment thresholds chosen for initiation and stop of the CSII may be adapted with developing technology and increasing competence of the nursing and medical teams.
Availability of data and materials
All analyzed data are included in this article. Further inquiries can be directed to the corresponding author.
Change history
21 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s44253-023-00011-4
References
Hay WW, Rozance PJ (2018) Neonatal hyperglycemia-causes, treatments, and cautions. J Pediatr 200:6–8. https://doi.org/10.1016/j.jpeds.2018.04.046
Zamir I, Tornevi A, Abrahamsson T, Ahlsson F, Engström E, Hallberg B, Hansen-Pupp I, Sjöström ES, Domellöf M (2018) Hyperglycemia in extremely preterm infants-insulin treatment, mortality and nutrient intakes. J Pediatr 200:104-110.e1. https://doi.org/10.1016/j.jpeds.2018.03.049
Lemelman MB, Letourneau L, Greeley SAW (2018) Neonatal diabetes mellitus: an update on diagnosis and management. Clin Perinatol 45:41–59. https://doi.org/10.1016/j.clp.2017.10.006
Hey E (2005) Hyperglycaemia and the very preterm baby. Semin Fetal Neonatal Med 10:377–387. https://doi.org/10.1016/j.siny.2005.04.008
Hays SP, Smith EO, Sunehag AL (2006) Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics 118:1811–1818. https://doi.org/10.1542/peds.2006-0628
Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA (2006) Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol 26:730–736. https://doi.org/10.1038/sj.jp.7211593
Heimann K, Peschgens T, Kwiecien R, Stanzel S, Hoernchen H, Merz U (2007) Are recurrent hyperglycemic episodes and median blood glucose level a prognostic factor for increased morbidity and mortality in premature infants </=1500 g? J Perinat Med 35:245–248. https://doi.org/10.1515/JPM.2007.057
Rowen JL, Atkins JT, Levy ML, Baer SC, Baker CJ (1995) Invasive fungal dermatitis in the < or = 1000-gram neonate. Pediatrics 95:682–687
Manzoni P, Castagnola E, Mostert M, Sala U, Galletto P, Gomirato G (2006) Hyperglycaemia as a possible marker of invasive fungal infection in preterm neonates. Acta Paediatr 95:486–493. https://doi.org/10.1080/08035250500444867
Garg R, Agthe AG, Donohue PK, Lehmann CU (2003) Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol 23:186–194. https://doi.org/10.1038/sj.jp.7210879
Blanco CL, Baillargeon JG, Morrison RL, Gong AK (2006) Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol 26:737–741. https://doi.org/10.1038/sj.jp.7211594
Ertl T, Gyarmati J, Gaál V, Szabó I (2006) Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biol Neonate 89:56–59. https://doi.org/10.1159/000088199
Sinclair JC, Bottino M, Cowett RM (2011) Interventions for prevention of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst Rev CD007615. https://doi.org/10.1002/14651858.CD007615.pub3
Bottino M, Cowett RM, Sinclair JC (2011) Interventions for treatment of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst Rev CD007453. https://doi.org/10.1002/14651858.CD007453.pub3
Tubiana-Rufi N (2007) Insulin pump therapy in neonatal diabetes. Endocr Dev 12:67–74. https://doi.org/10.1159/000109606
Beardsall K, Pesterfield CL, Acerini CL (2011) Neonatal diabetes and insulin pump therapy. Arch Dis Child Fetal Neonatal Ed 96:F223-224. https://doi.org/10.1136/adc.2010.196709
Park JH, Kang JH, Lee K-H, Kim N-H, Yoo H-W, Lee D-Y, Yoo E-G (2013) Insulin pump therapy in transient neonatal diabetes mellitus. Ann Pediatr Endocrinol Metab 18:148–151. https://doi.org/10.6065/apem.2013.18.3.148
Bharucha T, Brown J, McDonnell C, Gebert R, McDougall P, Cameron F, Werther G, Zacharin M (2005) Neonatal diabetes mellitus: Insulin pump as an alternative management strategy. J Paediatr Child Health 41:522–526. https://doi.org/10.1111/j.1440-1754.2005.00696.x
Desenfants A, Soleirol M, Salet R, Castro FB, Guillou CL, Maio MD, Tran TA (2022) Efficiency of continuous subcutaneous insulin infusion for premature neonate: a case report. NEO 119:260–263. https://doi.org/10.1159/000521695
Muzzy Williamson JD, Thurlow B, Mohamed MW, Yokom D, Casas L (2020) Neonatal hyperglycemia in a preterm infant managed with a subcutaneous insulin pump. Am J Health Syst Pharm 77:739–744. https://doi.org/10.1093/ajhp/zxaa056
Anderson de la Llana S, Klee P, Santoni F, Stekelenburg C, Blouin J-L, Schwitzgebel VM (2015) Gene variants associated with transient neonatal diabetes mellitus in the very low birth weight infant. Horm Res Paediatr 84:283–288. https://doi.org/10.1159/000437378
Das-Kundu S, Fontijn J, Mönkhoff M, Neumann R, Szinnai G, Schulzke S (2020) Prevention and treatment of hypoglycaemia in neonates with a gestational age from 35 0/7 weeks in maternity wards. Paediatrica 32:1–2021. https://doi.org/10.35190/f2021.1.5
Zahid N, Taylor KMG, Gill H, Maguire F, Shulman R (2008) Adsorption of insulin onto infusion sets used in adult intensive care unit and neonatal care settings. Diabetes Res Clin Pract 80:e11–13. https://doi.org/10.1016/j.diabres.2008.02.013
Sevick SH. Compatibility of various pharmaceutical agents with Pall Supor intravenous filter devices. Technical Report, Pall Ann Arbor, 2001
Fuloria M, Friedberg MA, DuRant RH, Aschner JL (1998) Effect of flow rate and insulin priming on the recovery of insulin from microbore infusion tubing. Pediatrics 102:1401–1406. https://doi.org/10.1542/peds.102.6.1401
Managing Hazardous Material Incidents (MHMI). Volumes III. Agency for Toxic Substances and Disease Registry (ATSDR). 2001. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/MHMI/mmg115.pdf. Accessed 30 Jan 2023
Begum-Hasan J, Bruce AAK, Koster J (2010) Case study: experience in insulin pump therapy during the neonatal period. Clinical Diabetes 28:30–33. https://doi.org/10.2337/diaclin.28.1.30
Bettini R, Cocconi D (2001) Handbook of pharmaceutical excipients, Third Edition: Arthur H. Kibbe (ed.), Pharmaceutical Press, London, 2000, 665 pp. https://doi.org/10.1016/S0168-3659(01)00243-7
Accuchek spirit combo user-guide. https://www.rochediabetescaremea.com/sites/g/files/iut446/f/accu-chek-spirit-combo-user-guide-en.pdf. Accessed 30 Jan 2023
Acknowledgements
The authors would like to thank Lucie Bouchoud and Sandrine Fleury-Souverain for their contribution to the development of the diluted insulin solution.
Code availability
Not applicable.
Funding
Open access funding provided by University of Geneva. No sponsors were involved in our research.
Author information
Authors and Affiliations
Contributions
Andrea Becocci, Nathalie Bochaton, Sébastien Fau, Philippe Klee, Luz Perrenoud, Caroline Fonzo-Christe, and Riccardo E. Pfister contributed to the writing of this article. Andrea Becocci and Riccardo E. Pfister conceived and supervised the study. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The procedures and treatment were discussed with and approved by the parents of all the patients. Written informed consent was obtained from parents for the study and publication of this case series and any accompanying images. This study protocol was reviewed and approved by the Cantonal Ethics Commission for Research on Human Beings (CCER) in Geneva, approval number 2022–01179.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been updated to correct figure 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becocci, A., Bochaton, N., Fau, S. et al. Continuous subcutaneous insulin infusion via an insulin pump in extremely premature neonates—a case series. Intensive Care Med. Paediatr. Neonatal 1, 3 (2023). https://doi.org/10.1007/s44253-023-00004-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-023-00004-3