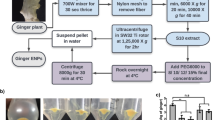
Abstract
Extracellular vesicles (EVs) are nanoscale entities secreted by various cells, encapsulating various nucleic acids and proteins that play important roles in cellular activities. Although rice bran is known for its richness in phytochemicals such as tocopherol and tocotrienol, the distribution of these compounds within EVs has not been extensively studied. The objective of this study was to detect and analyze the presence of vitamin E in EVs extracted from rice bran. We investigated several EV extraction methods, including rotation, vortex mixing, and ultrasonication, followed by post-extraction techniques such as ultracentrifugation, ultrafiltration, and lyophilization. Vitamin E in the EVs from rice bran was analyzed using LC-FLD. This study is the first to identify tocopherol and tocotrienol in rice bran-derived EVs. Our results indicate that ultracentrifugation followed by rotation is the most effective method for the preparation of rice bran-derived EVs. Notably, the vitamin E profile in EVs varies depending on the preparation method and differs from that in rice bran extracts. The pronounced presence of vitamin E in EVs suggests unique pharmacokinetics and underscores the potential of EVs as carriers for drug delivery systems. This study not only confirms the presence of vitamin E in EVs, but also underscores the potential of EVs and their phytochemical content for therapeutic applications.
Graphical abstract





Similar content being viewed by others
Abbreviations
- EVs:
-
Extracellular vesicles
- T3s:
-
Tocotrienols
- Tocs:
-
Tocopherols
- NTA:
-
Nano-tracking analysis
- LC-FLD:
-
Liquid chromatography with fluorescence detector
- SEM:
-
Scanning electron microscopy
References
C.A. Rohrer, T.J. Siebenmorgen, Biosyst. Eng. (2004). https://doi.org/10.1016/j.biosystemseng.2004.04.009
E. Serbinova, V. Kagan, D. Han, L. Packer, Free Radic. Biol. Med. (1991). https://doi.org/10.1016/0891-5849(91)90033-y
K. Nesaretnam, N. Guthrie, A.F. Chambers, K.K. Carroll, Lipids (1995). https://doi.org/10.1007/BF02536615
K. Nesaretnam, R. Ambra, K.R. Selvaduray, A. Radhakrishnan, K. Reimann, G. Razak, F. Virgili, Lipids (2004). https://doi.org/10.1007/s11745-004-1251-1
A.A. Qureshi, W.C. Burger, D.M. Peterson, C.E. Elson, J. Biol. Chem. 261, 10544–10550 (1986)
M. Yáñez-Mó, P.R.-M. Siljander, Z. Andreu, A.B. Zavec, F.E. Borràs, E.I. Buzas, K. Buzas, E. Casal, F. Cappello, J. Carvalho, E. Colás, A. Cordeiro-da Silva, S. Fais, J.M. Falcon-Perez, I.M. Ghobrial, B. Giebel, M. Gimona, M. Graner, I. Gursel, M. Gursel, N.H.H. Heegaard, A. Hendrix, P. Kierulf, K. Kokubun, M. Kosanovic, V. Kralj-Iglic, E.-M. Krämer-Albers, S. Laitinen, C. Lässer, T. Lener, E. Ligeti, A. Linē, G. Lipps, A. Llorente, J. Lötvall, M. Manček-Keber, A. Marcilla, M. Mittelbrunn, I. Nazarenko, E.N.M. Nolte-’t Hoen, T.A. Nyman, L. O’Driscoll, M. Olivan, C. Oliveira, É. Pállinger, H.A. Del Portillo, J. Reventós, M. Rigau, E. Rohde, M. Sammar, F. Sánchez-Madrid, N. Santarém, K. Schallmoser, M.S. Ostenfeld, W. Stoorvogel, R. Stukelj, S.G. Van der Grein, M.H. Vasconcelos, M.H.M. Wauben, O. De Wever, J Extracell Vesicles (2015). https://doi.org/10.3402/jev.v4.27066.
C. Lei, Y. Teng, L. He, M. Sayed, J. Mu, F. Xu, X. Zhang, A. Kumar, K. Sundaram, M.K. Sriwastva, L. Zhang, S.-Y. Chen, W. Feng, S. Zhang, J. Yan, J.W. Park, M.L. Merchant, X. Zhang, H.-G. Zhang, iScience (2021). https://doi.org/10.1016/j.isci.2021.102511
B. Wang, X. Zhuang, Z.-B. Deng, H. Jiang, J. Mu, Q. Wang, X. Xiang, H. Guo, L. Zhang, G. Dryden, J. Yan, D. Miller, H.-G. Zhang, Mol. Ther. (2014). https://doi.org/10.1038/mt.2013.190
T. Umezu, M. Takanashi, Y. Murakami, S.-I. Ohno, K. Kanekura, K. Sudo, K. Nagamine, S. Takeuchi, T. Ochiya, M. Kuroda, Mol. Ther. Methods Clin. Dev. (2021). https://doi.org/10.1016/j.omtm.2021.03.006
Y. Akao, Y. Kuranaga, K. Heishima, N. Sugito, K. Morikawa, Y. Ito, T. Soga, T. Ito, J. Nutr. Biochem. (2022). https://doi.org/10.1016/j.jnutbio.2021.108922
S. Ju, J. Mu, T. Dokland, X. Zhuang, Q. Wang, H. Jiang, X. Xiang, Z.-B. Deng, B. Wang, L. Zhang, M. Roth, R. Welti, J. Mobley, Y. Jun, D. Miller, H.-G. Zhang, Mol. Ther. (2013). https://doi.org/10.1038/mt.2013.64
J. Mu, X. Zhuang, Q. Wang, H. Jiang, Z.-B. Deng, B. Wang, L. Zhang, S. Kakar, Y. Jun, D. Miller, H.-G. Zhang, Mol. Nutr. Food Res. (2014). https://doi.org/10.1002/mnfr.201300729
L. Alvarez-Erviti, Y. Seow, H. Yin, C. Betts, S. Lakhal, M.J.A. Wood, Nat. Biotechnol. (2011). https://doi.org/10.1038/nbt.1807
Q. Wang, X. Zhuang, J. Mu, Z.-B. Deng, H. Jiang, L. Zhang, X. Xiang, B. Wang, J. Yan, D. Miller, H.-G. Zhang, Nat. Commun. (2013). https://doi.org/10.1038/ncomms2886
A. Cheruvanky, H. Zhou, T. Pisitkun, J.B. Kopp, M.A. Knepper, P.S.T. Yuen, R.A. Star, Am. J. Physiol. Renal Physiol (2007). https://doi.org/10.1152/ajprenal.00434.2006
N. Zarovni, A. Corrado, P. Guazzi, D. Zocco, E. Lari, G. Radano, J. Muhhina, C. Fondelli, J. Gavrilova, A. Chiesi, Methods (2015). https://doi.org/10.1016/j.ymeth.2015.05.028
J. Folch, M. Lees, G.H.S. Stanley, J. Biol. Chem. (1957). https://doi.org/10.1016/s0021-9258(18)64849-5
P. Sookwong, K. Nakagawa, K. Murata, Y. Kojima, T. Miyazawa, J. Agric. Food Chem. (2007). https://doi.org/10.1021/jf0621572
J. Kim, S. Li, S. Zhang, J. Wang, Asian J. Pharm. Sci. (2022). https://doi.org/10.1016/j.ajps.2021.05.006
S.K. Channavajjhala, M. Rossato, F. Morandini, A. Castagna, F. Pizzolo, F. Bazzoni, O. Olivieri, Clin. Chem. Lab. Med. (2014). https://doi.org/10.1515/cclm-2013-0562
T. Yamashita, Y. Takahashi, M. Nishikawa, Y. Takakura, Eur. J. Pharm. Biopharm. (2016). https://doi.org/10.1016/j.ejpb.2015.10.017
S. Hagl, D. Berressem, B. Bruns, N. Sus, J. Frank, G.P. Eckert, Molecules (2015). https://doi.org/10.3390/molecules200916524
J. Atkinson, R.F. Epand, R.M. Epand, Free Radic. Biol. Med. (2008). https://doi.org/10.1016/j.freeradbiomed.2007.11.010
A.M. Abraham, S. Wiemann, G. Ambreen, J. Zhou, K. Engelhardt, J. Brüßler, U. Bakowsky, S.-M. Li, R. Mandic, G. Pocsfalvi, C.M. Keck, Pharmaceutics (2022). https://doi.org/10.3390/pharmaceutics14030476
R. Anusha, S. Priya, Mol. Nutr. Food Res. (2022). https://doi.org/10.1002/mnfr.202200142
M.S.A. Mutalib, H. Khaza’ai, K.W.J. Wahle, Food Res. Int. (2003). https://doi.org/10.1016/S0963-9969(02)00173-4
A. Theriault, Q. Wang, A. Gapor, K. Adeli, Arterioscler. Thromb. Vasc. Biol. (1999). https://doi.org/10.1161/01.atv.19.3.704
T. Nakatomi, M. Itaya-Takahashi, Y. Horikoshi, N. Shimizu, I.S. Parida, M. Jutanom, T. Eitsuka, Y. Tanaka, J.-M. Zingg, T. Matsura, K. Nakagawa, Sci. Rep. (2023). https://doi.org/10.1038/s41598-023-34584-z
Y. Saito, Y. Yoshida, K. Nishio, M. Hayakawa, E. Niki, Ann. N. Y. Acad. Sci. (2004). https://doi.org/10.1196/annals.1331.047
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest. Akio Iio is an employee and received a salary in Clea Japan Inc., but has no financial relationships to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takasu, S., Watanabe, R., Sugito, N. et al. Unveiling the vitamin E profile in rice bran extracellular vesicles: evaluation of extraction and preparation methods. ANAL. SCI. 40, 935–941 (2024). https://doi.org/10.1007/s44211-024-00550-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-024-00550-6