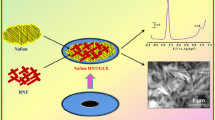
Abstract
Acetaminophen (AC) can inhibit the synthesis of prostaglandins in the body, and has antipyretic and analgesic effects. In this paper, a two-step microwave impregnation method was used to prepare anthraquinone (AQ)-doped carbon composite, which were applied to the surface modification of glassy carbon electrodes (GCE) for the determination of acetaminophen (AC) using differential pulse voltammetry (DPV). The composites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Raman and Fourier infrared spectroscopy (FT-IR). The results showed that anthraquinone was successfully modified on the surface of activated carbon. The peak current of AC increased with its concentration in the range of 0.1 μM to 700 μM (R2 = 0.998) and a detection limit of 0.05 μM was obtained with 20%AQ doped carbon electrochemical sensor (20%AQ-C/GCE). Electrochemical Impedance Spectroscopy (EIS) test results indicated that the charge transfer resistance (Rct) of 20%AQ-C/GCE is only the one-fourth of that of bare GCE. The proposed 20%AQ-C/GCE sensor has good stability, reproducibility and selectivity for the detection of AC. The sensor is also suitable for the detection of real samples, indicating its good practicality.
Graphical Abstract

















Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
A.S. Farag, Voltammetric determination of acetaminophen in pharmaceutical preparations and human urine using glassy carbon paste electrode modified with reduced graphene oxide. Anal. Sci. 38, 1213 (2022)
S. Shahrokhian, E. Asadian, Simultaneous voltammetric determination of ascorbic acid, acetaminophen and isoniazid using thionine immobilized multi-walled carbon nanotube modified carbon paste electrode. Electrochim. Acta 55, 666 (2010)
A. Pyka, M. Budzisz, M. Dolowy, Validation thin layer chromatography for the determination of acetaminophen in tablets and comparison with a pharmacopeial method. Biomed. Res. Int. 2013, 545703 (2013)
F.A. Mohamed, M.A. AbdAllah, S.M. Shmmat, Selective sepctrophotometric determination of p-aminophenol and acetaminophen. Talanta 44, 61 (1997)
Y.C. Qiu, L.Z. Benet, A.L. Burlingame, Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J. Biol. Chem. 273, 17940 (1998)
W.A. Garland, K.C. Hsiao, E.J. Pantuck, A.H. Conney, Quantitative determination of phenacetion and its metabolite acetaminophen by GLC-chemical ionization mass spectrometry. J. Pharm. Sci. 66, 340 (1977)
J.R. Bales, P.J. Sadier, J.K. Nicholson, J.A. Timbrell, Urinary Excretion of acetaminophen and its metabolites as studied by proton NMR spectroscopy. Clin. Chem. 30, 1631 (1984)
D. Kim, J.M. Kim, Y. Jeon, J. Lee, J. Oh, W.H. Antink, D. Kim, Y. Piao, Novel two-step activation of biomass-derived carbon for highly sensitive electrochemical determination of acetaminophen. Sens. Actuators B Chem. 259, 50 (2018)
N. Mohammadi, M. Bahmaei, A.M. Sharif, Highly sensitive CuZnO-Fe3O4/rGO modified glassy carbon electrode for the electrochemical determination of acetaminophen, tyrosine and codeine in human blood plasma and urine. J. Electroanal. Chem. 902, 115768 (2021)
L.J. Sun, Q. Li, M. Zheng, S.Y. Lin, C.L. Guo, L.Y. Luo, S. Guo, Y.X. Li, C. Wang, B.J. Jiang, Efficient Suzuki-Miyaura cross-coupling reaction by loading trace Pd nanoparticles onto copper-complex-derived Cu/C-700 solid support. J. Colloid Interface Sci. 608, 2463 (2022)
J. Balintova, R. Pohl, P. Horakova, P. Vidlakova, L. Havran, M. Fojta, M. Hocek, Anthraquinone as a redox label for DNA: synthesis, enzymatic incorporation, and electrochemistry of anthraquinone-modified nucleosides, nucleotides, and DNA. Chem. 17, 14063 (2011)
M. Wagner, K. Qvortrup, K.E. Grier, M.R. Ottosen, J.O. Petersen, D. Tanner, J. Ulstrup, J. Zhang, Gold-carbonyl group interactions in the electrochemistry of anthraquinone thiols self-assembled on Au (111)-surfaces. Chem. Sci. 10, 3927 (2019)
Y. Gao, W.H. Zhu, Y.Q. Li, J.L. Li, S.N. Yun, T.L. Huang, Novel porous carbon felt cathode modified by cyclic voltammetric electrodeposited polypyrrole and anthraquinone 2-sulfonate for an efficient electro-Fenton process. Int. J. Hydrog. Energy 46, 9707 (2021)
Y. Yang, S. Ciampi, M.H. Choudhury, J.J. Gooding, Light Activated electrochemistry: light intensity and pH dependence on electrochemical performance of anthraquinone derivatized silicon. J. Phys. Chem. C 120, 2874 (2016)
K. Nueangnoraj, T. Tomai, H. Nishihara, T. Kyotani, I. Honma, An organic proton battery employing two redox-active quinones trapped within the nanochannels of zeolite-templated carbon. Carbon 107, 831 (2016)
W.H. Zhu, Y.Q. Li, Y. Gao, C. Wang, J.F. Zhang, H.L. Bai, T.L. Huang, A new method to fabricate the cathode by cyclic voltammetric electrodeposition for electro-Fenton application. Electrochim. Acta 349, 136415 (2020)
A. Le Comte, T. Brousse, D. Bélanger, Simpler and greener grafting method for improving the stability of anthraquinone-modified carbon electrode in alkaline media. Electrochim. Acta 137, 447 (2014)
F.Y. Yu, K. Wang, C. Wang, X.X. He, Y. Liao, S.L. Zhao, H. Mao, X.T. Li, J. Ma, Anthraquinone covalently modified carbon nanotubes for efficient and steady electrocatalytic H2O2 generation. Chem. Res. Chin. Univ. 36, 1332 (2020)
X. Liu, C.L. Zhong, J. Ji, W. Yang, Z.F. Tian, Y.C. Chen, Q.F. Tian, Polyoxometalate/carbon black modified glassy carbon electrode for the detection of dopamine. Electroanalysis 35, 116 (2023)
Q. F. Tian, B. Fu, Electrocatalytic Performance of Nitrogen and Phosphorus Co-Doped Carbon Supported Pd Catalysts for Formic Acid Oxidation, J. Comp. Chem. 2, (2018)
X. Chen, H.W. Wang, H. Yi, X.F. Wang, X. Yan, Z.H. Guo, Anthraquinone on porous carbon nanotubes with improved supercapacitor performance. J. Phys. Chem. C 118, 8262 (2014)
X.L. Gao, D.F. Du, S. Li, X. Yan, W. Xing, P. Bai, Q.Z. Xue, Z.F. Yan, Outstanding capacitive performance of ordered mesoporous carbon modified by anthraquinone. Electrochim. Acta 259, 110 (2018)
J. Ma, F.Y. Yu, D.Q. Hu, Q.C. Wang, L. Li, W. Zhang, Anthraquinone covalently modified carbon fiber with significantly improved electrocatalytic performance for hydrogen peroxide production. J. SW. Minzu Univ. 47, 488 (2021)
H.J. Wang, S.Y. Zhang, S.F. Li, J.Y. Qu, Electrochemical sensor based on palladium-reduced graphene oxide modified with gold nanoparticles for simultaneous determination of acetaminophen and 4-aminophenol. Talanta 178, 188 (2018)
M.C.N. Ngwem, J.C. Kemmegne-Mbouguen, H.W. Langmi, N.M. Musyoka, R. Mokaya, Electrochemical sensor for ascorbic acid, acetaminophen and nitrite based on organoclay/Zr‐MOF film modified glassy carbon electrode. ChemistrySelect 7, 2308 (2022)
D.X. Yang, L.D. Zhu, X.Y. Jiang, L.P. Guo, Sensitive determination of Sudan I at an ordered mesoporous carbon modified glassy carbon electrode. Sens. Actuators B Chem. 141, 124 (2009)
K. Reddaiah, T.M. Reddy, K. Mallikarjuna, G. Narasimha, Electrochemical detection of dopamine at poly (solochrome cyanine)/Pd nanoparticles doped modified carbon paste electrode and simultaneous resolution in the presence of ascorbic acid and uric acid: a voltammetric method. Anal. Methods 5, 5627 (2013)
J.F. Gao, J.F. Hou, L.B. Kong, Capacitive charge storage mechanism in sanmartinite to be determined by qualitative and quantitative electrochemical analysis. Electrochim. Acta 439, 141692 (2023)
W.C. Liang, L.L. Liu, Y.G. Li, H.L. Ren, T.T. Zhu, Y.W. Xu, B.C. Ye, Nitrogen-rich porous carbon modified electrochemical sensor for the detection of acetaminophen. J. Electroanal. Chem. 855, 113496 (2019)
A. Dehnavi, A. Soleymanpour, Highly sensitive voltammetric electrode for the trace measurement of methyldopa based on a pencil graphite modified with phosphomolibdate/graphene oxide. Microchem. J. 157, 104969 (2020)
W.Q. Zhang, L.K. Zong, S.Q. Liu, S. Pei, Y.S. Zhang, X.M. Ding, B. Jiang, Y.P. Zhang, An electrochemical sensor based on electro-polymerization of caffeic acid and Zn/Ni-ZIF-8-800 on glassy carbon electrode for the sensitive detection of acetaminophen. Biosens. Bioelectron. 131, 200 (2019)
M. Ali, S. Sharma, R. Singh, K. Sharma, S. Majhi, D. Guin, C.S.P. Tripathi, Barium titanate nanocubes as a dual electrochemical sensor for detection of dopamine and acetaminophen. J. Electrochem. Soc. 169, 067512 (2022)
S.P. Li, J.Y. Zhou, M. Noroozifar, K. Kerman, Gold–platinum core-shell nanoparticles with thiolated polyaniline and multi-walled carbon nanotubes for the simultaneous voltammetric determination of six drug molecules. Chemosensors 9, 24 (2021)
E.K. Savan, Electrochemical determination of N-acetyl cysteine in the presence of acetaminophen at multi-walled carbon nanotubes and nafion modified sensor. Sens. Actuators B Chem. 282, 500 (2019)
S. Lotfi, H. Veisi, Pd nanoparticles decorated poly-methyldopa@GO/Fe(3)O(4) nanocomposite modified glassy carbon electrode as a new electrochemical sensor for simultaneous determination of acetaminophen and phenylephrine. Mater Sci Eng C Mater Biol Appl 105, 110112 (2019)
M. Amiri-Aref, J.B. Raoof, R. Ojani, Electrocatalytic oxidation and selective determination of an opioid analgesic methadone in the presence of acetaminophen at a glassy carbon electrode modified with functionalized multi-walled carbon nanotubes: application for human urine, saliva and pharmaceutical samples analysis. Colloids Surf. B 109, 287 (2013)
M.D. Tezerjani, A. Benvidi, A. Dehghani Firouzabadi, M. Mazloum-Ardakani, A. Akbari, Epinephrine electrochemical sensor based on a carbon paste electrode modified with hydroquinone derivative and graphene oxide nano-sheets: simultaneous determination of epinephrine, acetaminophen and dopamine. Measurement 101, 183 (2017)
Acknowledgements
This work was supported by the Wuhan Institute of Technology Graduate Education Innovation Fund (CX2022027), the financial supports from the Opening Research Fund of Hubei Key Laboratory for Processing and Application of Catalytic Materials and the Natural Science Foundation of Hubei Province (Grant No. 2016CFA079)
Funding
Natural Science Foundation of Hubei Province, 2016CFA079, Qifeng Tian.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, C., Chen, Y., Zheng, Y. et al. Anthraquinone/activated carbon electrochemical sensor and its application in acetaminophen analysis. ANAL. SCI. (2024). https://doi.org/10.1007/s44211-024-00537-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44211-024-00537-3