Abstract
We report on the development of a versatile and accurate bioanalytical method for bevacizumab using a pretreatment method combining affinity purification with anti-idiotypic DNA aptamers and centrifugal ultrafiltration concentration, followed by liquid chromatography (LC)-fluorescence analysis. An affinity purification method using Sepharose beads as an affinity support removed immunoglobulin G and a large amount of coexisting substances in the serum sample. Purified bevacizumab was separated as a single peak by conventional LC and detected fluorometrically, showing good linearity (R2 = 0.999) in the range of 5–200 μg/mL, sufficient to analyze bevacizumab concentrations in the blood of bevacizumab-treated patients. By combining this purification method with a concentration method using a centrifugal filtration device that inhibits non-specific adsorption of bevacizumab, the quantitative range was reduced by a factor of 10 while showing good linearity (R2 = 0.999) in the 0.5–20 μg/mL range. The developed analytical method is expected to be used not only for general bioanalysis of therapeutic mAbs in clinical settings, but also for next-generation antibody drugs that show drug efficacy at low concentrations and for analysis of trace samples.
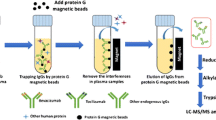
Graphical abstract





Similar content being viewed by others
Data availability
Data will be made available on request.
References
T. Suzuki, A. Ishii-Watabe, M. Tada, T. Kobayashi, T. Kanayasu-Toyoda, T. Kawanishi, T. Yamaguchi, J. Immunol. 184, 1968 (2010). https://doi.org/10.4049/jimmunol.0903296
F. Darrouzain, S. Bian, C. Desvignes, C. Bris, H. Watier, G. Paintaud, A. de Vries, Ther. Drug Monit. 39, 316 (2017). https://doi.org/10.1097/FTD.0000000000000419
C.W.N. Damen, J.H.M. Schellens, J.H. Beijnen, Hum. Antibodies 18, 47 (2009). https://doi.org/10.3233/HAB-2009-0206
J.W. Lee, M. Kelley, L.E. King, J. Yang, H. Salimi-Moosavi, M.T. Tang, J.-F. Lu, J. Kamerud, A. Ahene, H. Myler, C. Rogers, AAPS J. 13, 99 (2011). https://doi.org/10.1208/s12248-011-9251-3
E. Ezan, F. Bitsch, Bioanalysis 1, 1375 (2009). https://doi.org/10.4155/bio.09.121
R.F. Staack, J.O. Stracke, K. Stubenrauch, R. Vogel, J. Schleypen, A. Papadimitriou, Bioanalysis 3, 523 (2011). https://doi.org/10.4155/bio.11.16
K. Todoroki, H. Mizuno, E. Sugiyama, T. Toyo’oka, J. Pharm. Biomed. Anal. 179, 112991 (2020). https://doi.org/10.1016/j.jpba.2019.112991
K.A.M. de Jong, S.J. van Breugel, M.J.X. Hillebrand, H. Rosing, A.D.R. Huitema, J.H. Beijnen, Bioanalysis 12, 1405 (2020). https://doi.org/10.4155/bio-2020-0204
K. Todoroki, T. Yamada, H. Mizuno, T. Toyo’oka, Anal. Sci. 34, 397 (2018). https://doi.org/10.2116/analsci.17R003
C. Wei, D. Su, J. Wang, W. Jian, D. Zhang, Curr. Pharmacol. Rep. 4, 45 (2018). https://doi.org/10.1007/s40495-017-0118-x
N. Iwamoto, T. Shimada, Pharmacol. Ther. 185, 147 (2018). https://doi.org/10.1016/j.pharmthera.2017.12.007
C.W.N. Damen, E.J.B. Derissen, J.H.M. Schellens, H. Rosing, J.H. Beijnen, J. Pharm. Biomed. Anal. 50, 861 (2009). https://doi.org/10.1016/j.jpba.2009.04.031
T.M. Dillon, P.V. Bondarenko, M. Speed Ricci, J. Chromatogr. A 1053, 299 (2004). https://doi.org/10.1016/j.chroma.2004.08.058
T.M. Dillon, P.V. Bondarenko, D.S. Rehder, G.D. Pipes, G.R. Kleemann, M.S. Ricci, J. Chromatogr. A 1120, 112 (2006). https://doi.org/10.1016/j.chroma.2006.01.016
T. Yamada, H. Mizuno, J.Z. Min, T. Toyo’oka, K. Todoroki, Chromatography 39, 21 (2018). https://doi.org/10.15583/jpchrom.2017.014
S. Sotomatsu, T. Yamada, H. Mizuno, H. Hayashi, T. Toyo’oka, K. Todoroki, Chromatography 40, 99 (2019). https://doi.org/10.15583/jpchrom.2019.013
K. Todoroki, T. Nakano, Y. Eda, K. Ohyama, H. Hayashi, D. Tsuji, J.Z. Min, K. Inoue, N. Iwamoto, A. Kawakami, Y. Ueki, K. Itoh, T. Toyo’oka, Anal. Chim. Acta 916, 112 (2016). https://doi.org/10.1016/j.aca.2016.02.029
T. Saito, Y. Shimizu, K. Tsukakoshi, K. Abe, J. Lee, K. Ueno, R. Asano, B.V. Jones, T. Yamada, T. Nakano, J. Tong, A. Hishiki, K. Hara, H. Hashimoto, K. Sode, T. Toyo’oka, K. Todoroki, K. Ikebukuro, Biosens. Bioelectron. 203, 114027 (2022). https://doi.org/10.1016/j.bios.2022.114027
T. Yamada, T. Saito, Y. Hill, Y. Shimizu, K. Tsukakoshi, H. Mizuno, H. Hayashi, K. Ikebukuro, T. Toyo’oka, K. Todoroki, Anal. Chem. 91, 3125 (2019). https://doi.org/10.1021/acs.analchem.8b05725
T. Yamada, T. Saito, Y. Shimizu, K. Tsukakoshi, H. Hayashi, H. Mizuno, D. Tsuji, K. Yamamoto, K. Itoh, T. Toyo’oka, K. Ikebukuro, K. Todoroki, Molecules 24, 857 (2019). https://doi.org/10.3390/molecules24050857
Y. Shibata, T. Yamada, E. Sugiyama, H. Mizuno, Chromatography 41, 123 (2020). https://www.jstage.jst.go.jp/article/jpchrom/41/3/41_2020.016/_article/-char/ja/.
U.S. FDA, Bioanalytical Method Validation, Guidance for Industry (US Department Of, Rockville, 2018)
A.C. Moser, D.S. Hage, Bioanalysis 2, 769 (2010). https://doi.org/10.4155/bio.10.31
Q. Zhao, X.-F. Li, Y. Shao, X.C. Le, Anal. Chem. 80, 7586 (2008). https://doi.org/10.1021/ac801206s
E. Bergsland, M.N. Dickler, Oncologist 9(Suppl 1), 36 (2004). https://doi.org/10.1634/theoncologist.9-suppl_1-36
K. Todoroki, J. Tong, M. Aoki, N. Kobayashi, R. Isobe, H. Tasaki, T. Yamada, A. Furusho, E. Sugiyama, H. Mizuno, H. Hayashi, T. Toyo’oka, J. Pharm, Biomed. Anal. Open 1, 100006 (2023). https://doi.org/10.1016/j.jpbao.2023.100006
Acknowledgements
This work was supported by JSPS KAKENHI, Grant Nos. 16K08200, 19H03360, 22K06550, and the TaNeDS project funded by Daiichi-Sankyo Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Todoroki, K., Hamada, D., Yamada, T. et al. Development of a liquid chromatography-based versatile bioanalysis for bevacizumab based on pretreatment combining aptamer affinity purification and centrifugal ultrafiltration concentration. ANAL. SCI. 39, 1805–1811 (2023). https://doi.org/10.1007/s44211-023-00417-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00417-2