Abstract
Selenium (Se) is essential for human and animal nutrition, playing a key role in antioxidant and immune functions. Organic Se is better for supplementation because it is more efficiently assimilated and less toxic than its inorganic form. Due to the scarcity of Se in European soils, supplementation in feed and food is necessary. Currently, inorganic Se (sodium selenite and selenate) and organic Se in Se-enriched yeast are approved by the European Food Safety Authority (EFSA) to address Se deficiency. However, Se-enriched microalgae present a promising alternative. By supplementing their growth media with Se, microalgae convert it into organic forms like Se-cysteine and Se-methionine, creating Se-enriched biomass. This biomass can serve as a valuable Se source with the additional benefits of microalgae. This review evaluates the viability of microalgae as a Se supplementation vehicle in food and feed and explores its commercial applications in the European Union (EU), along with emerging projects and innovations in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Selenium (Se) is crucial for the organism’s antioxidant defence mechanism: it triggers the production of selenoproteins and contributes to the protection of cells against ROS (reactive oxygen species) and modulates the immune and reproductive system activities [1].
Soils used in European agriculture are commonly identified as having low Se levels [2]. As a result, meeting daily Se requirements through diet can be difficult [3]. Supplementation of food and feed has primarily involved the use of inorganic Se salts. However, this supplementation method with sodium selenite or selenate is associated with drawbacks, such as toxicity and low absorption rates within the body [4, 5]. In this context, biofortified food and feedstuffs, like crop products cultivated in Se-fertilised soils or Se fortified supplements, have been addressing Se insufficiency-related conditions [6, 7]. More recently, to address this problem, organic Se-enriched foods have been widely developed. These foods mainly include Se-enriched plants, animals, and microorganisms [3]. The primary forms present are selenomethionine (SeMet) and selenocysteine (SeCys). Organisms assimilate organic compounds at a rate of approximately 85–95%, whereas the assimilation rate for inorganic forms is around 40–50% [8,9,10].
The narrow margin between Se deficiency and toxicity underscores the importance of conducting comprehensive studies of Se-vehicle organisms, such as microalgae [4, 9, 11]. Recently, Se-enriched microalgae biomass has emerged as a potential source of organically bound Se that is highly accessible and bioavailable [3, 7]. Microalgae demonstrate efficient incorporation of Se, converting it into less hazardous organic forms, including Se-proteins, Se-amino acids (Se-AAs), Se-polysaccharides (Se-PSs), and Se-volatile compounds. Some studies have shown that the organically bonded Se is more suitable for human consumption compared to Se salts [3, 7, 9,10,11]. Therefore, the striking ability of microalgae to metabolise inorganic Se into low toxic reduced molecules offers a great Se-supplementation alternative, highlighting their potential to serve as a biological vehicle for Se-AAs [4, 9].
This review addresses microalgae-driven Se supplementation for food and feed with an overview of commercial applications and other alternative sources under the European Union (EU). This article provides a comprehensive overview of the latest literature on Se supplementation for dietary use in both humans and animals.
2 Importance of microalgae in food and feed
Microalgae are abundant in protein (~ 40–50%) and lipids (~ 15–30%) [12, 13] (Table 1). Regarding their fatty acid profile, microalgae can be a potential source of polyunsaturated fatty acids (PUFAs), which humans usually consume through fish, like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Interestingly, European infant milk formulas already contain microalgae purified, DHASCO® [14].
Microalgae proteins can provide a complete profile of essential amino acids (in contrast with most plant-derived proteins), which is crucial for the immune system [15]. These organisms also provide carbohydrates (~ 10–30%, Table 1) (starch, glycogen, and cellulose) and pigments (chlorophylls, β-carotene, lutein, and astaxanthin), which are high-value compounds [16]. Most extracts also have probiotic activity and a positive influence on the composition of gastrointestinal microflora [17, 18].
The ash content of microalgae depends on the concentration of minerals in the environment (growth conditions), which is why marine species like Nannochloropsis sp. show higher content of sodium compared to the freshwater Chlorella vulgaris. The literature reports slightly different values as well as for the nutrients [20]. Microalgae biomass also presents some minerals such as calcium (Ca), magnesium (Mg), phosphorus (P), potassium (K), sodium (Na), and iron (Fe) (Table 1) [15, 19,20,21,22]. These minerals are crucial for human body functions, making microalgae a valuable candidate for dietary supplements. The mineral absorption is related to the protein content, especially Fe which is a cofactor in protein metabolism, as well as Cu [23, 24]. Mg and P are also needed for protein formation, as well as Se and Zn which are important parts of the structure of many enzymes [24]. In a recently published study [25], the relationship between algal protein content and Se accumulation was evaluated for the microalgae, Parachlorella kessleri, Chlorella vulgaris, and Raphidocelis subcapitata. The algae were exposed to selenate and analysed for total protein and Se content during both exponential and stationary growth phases. No relationship was found between protein content and Se accumulation during the exponential phase, but a strong relationship was observed during the stationary phase across the species. From the obtained results, the authors suggested that cellular protein content in microalgae influences Se bioconcentration.
These microorganisms can produce the same amount of energy in smaller areas compared to higher plants [16]. Microalgae also allow the utilization of wastewater, or industrial side streams and exhaust gases, without competing for arable land [16]. Therefore, they might decrease the pressure on terrestrial food crops and water resources [15]. Nevertheless, its cultivation can be expensive, and algal biomass must be analysed for its composition and toxic compounds [26]. Chlorella sp. (e.g., C. pyrenoidosa, C. vulgaris, and C. luteoviridis) and Arthrospira platensis were commercially available and consumed as food ingredients prior to 1997 [10]. The EU list of novel foods includes Haematococcus pluvialis, Odontella aurita, Schizochytrium sp., Tetraselmis chui, and Ulkenia sp., among others [16, 17, 27]. Microalgae biomass is Generally Regarded As Safe (GRAS) for human consumption and shows no adverse effects on human health [16]. For instance, Chlorella is widely used as food supplements in several countries [17].
3 Selenium in human and animal nutrition: essentiality and toxicity
The content of Se in food products in each geographical region is proportional to its amount in the soil [28]. The amount of Se in the soil is primarily affected by the type of parent material and the climate: soils in humid and irrigated areas typically have lower Se levels because this mineral is washed away [2]. This could be why nearly all European nations are categorized as regions with low Se levels due to the scarcity of this mineral in agricultural lands [2]. Agricultural soils often suffer from Se deficiency, a condition likely to intensify with prolonged drought periods [2]. Depending on the dose, Se can be considered an essential micronutrient or toxic mineral. Essentiality has not yet been established for plants; however, this element is indispensable for many organisms, including mammals, bacteria, and archaea [29]. It has antioxidant and chemoprotective functions. Its deficiency may reduce growth and cause severe diseases in humans and livestock. Se nutritional functions are achieved by 25 Se-proteins in humans [1].
The element Se can exist in four oxidative states: selenium (0), selenide (− 2), selenite (+ 4), and selenate (+ 6) [9, 11]. In water, inorganic Se can be present as selenate (SeO42−) and selenite (SeO32−) [30]. Microorganisms’ biomethylation of Se, along with the decomposition of Se-rich organic matter, generates volatile Se compounds like dimethylselenium (DMSe), hydrogen selenide (H2Se), and selenium oxide (SeO2) [28].
Inorganic forms can be converted to organic forms like Se-AAs, Se-proteins, Se-PSs, Se-enzymes, and methyl-selenides) [30]. The most common Se-AAs are selenocysteine (SeCys-C3H6NO2Se), a derivative of cysteine, and selenomethionine (SeMet- C5H11NO2Se) [31]. Se-proteins are not fully characterized, among them the following can be identified: W, likely involved in muscle metabolism; S, regulates redox balance and R that has likely functions as an antioxidant [31].
Se plays a very important role in maintaining various physiological processes in the human body [32]. It shields cells from damage by oxidative stress and affects how the immune system operates [28] Its intake at low levels has been associated with beneficial effects, offering protection against various chronic diseases [7, 33, 34]. This trace mineral is relevant for viral infections since the immune system relies on a set of specific Se-proteins containing SeCys necessary for their expression and enzymatic processes, such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) [34,35,36]. Related to immune system, Se enhances the production of antibodies (IgG and IgM) and elevates the activity of T cells and macrophages. It helps slow down the aging process and accelerates cell regeneration [31, 32]. Several antioxidant enzymes rely on Se, like thioredoxin reductase which is involved in regulating the redox state of cells. This enzyme reduces thioredoxin which shows activity as growth factors, apoptosis inhibitors, and enzymes that catalyse hydroperoxide reduction. Se is also a component of iodotyronine deiodinase, which catalyzes the main biologically active thyroid hormone, triiodothyronine [31]. Iodothyronine deiodinase is an enzyme that needs Se and plays an important role in the regulation of thyroid hormones, responsible for proper development, growth, and cell metabolism [31].
SeMet exhibits greater bioavailability for metabolic functions due to its facile integration into proteins, and makes Se bioavailable for humans serving as a precursor to methylselenol, a potent anticarcinogen that restrains tumour invasion [35, 37]. Se inhibits tumor cell proliferation via the p53 tumor suppressor gene and Bcl-2 apoptosis-supressor gene. In fact, the anticancer properties of Se are primarily linked to its well-known antioxidant activity, the stabilizing effect on DNA, and the enhancement of cellular immune response. While the role of antioxidant mechanisms in cancer defense is crucial, it’s important to emphasize the significant effect of Se on the cell-killing activity of natural killer cells [31].
Nutrition represents the primary source of Se, essential for Se-enzymes activity [3, 38]. Furthermore, the intake of Se depends mainly on the type of food/feed consumed [3, 7]. Eggs, fish, corn, meat, vegetables (especially garlic) and Brazil nuts are Se-rich food sources [5].
The significance of dietary Se for health is not only dependent on the total intake of this mineral, but also the different Se species ingested [3]. Se is present in food in various forms: SeMet, the most common (plant sources, Se yeast, and other Se supplements), is also a predominant form of Se in feed ingredients [39, 40]; SeCys (feed); selenoneine (major Se compound in fish); selenomethylcysteine (MeSeCys) (plant sources, Se yeast); sodium selenite and selenate (dietary supplements, water supplies, fish, and plant sources) [3, 7].
Animal protein is the principal dietary source of Se for humans (~ 70%) [41]. Se intake from fruits and vegetables is notably lower as estimated in Table 2 [7, 41,42,43].
Vegetables contribute less than 8% of Se total intake [42, 44]. Therefore, there is a need to look for vegan alternatives.
The concentration and availability of Se in the food items are relatively low, necessitating consumption in larger quantities to meet Se intake requirements [45]. Se uptake of 5 µg kg−1 day−1 is the maximum recommended dose [9, 46]. The absolute daily Se requirement for an adult is approximately 40 µg, about 300 μg day−1 reduces cancer risks; and above 800 µg day−1 is considered toxic [9, 39, 42, 46]. Feed items are regularly fortified with diverse Se supplements up to the authorized threshold of 0.5 mg Se kg−1 in the EU [39, 47]. Typically, diets providing Se levels ranging from 0.1 to 0.3 mg kg−1 are generally sufficient to meet Se requirements for various animals [39]. Recommended daily intake of Se for animals is listed in the Hosnedlova et al. [48] review: for instance, 0.15–0.30 mg kg−1 for pigs and 0.30 mg kg−1 of DM for dairy cattle.
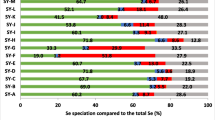
Table 3 shows China has the lowest Se intake in the Keshan area. Se levels for Portugal are below the recommended values, although not as low as in Finland before the treatment with Se fertilizer. Compared to these countries, the USA has a high daily Se intake [42, 43, 49, 50].
In various regions of China, recent research has indicated that individuals diagnosed with COVID-19 demonstrate reduced Se blood levels [7, 51, 52]: most studies measured serum Se levels, and one measured urinary Se level, as well as GSH-Px [53]. Nonetheless, there is a lack of information regarding the Se status of individual patients severely impacted by this illness. Se might have a role in protection against COVID infection [52], and its deficiency could be considered an indicator of the severity, mortality, and overall risk of COVID-19 [53].
Keshan disease is associated with necrotic lesions throughout the myocardium, and in 1935, an outbreak occurred in China. It was verified that Chinese populations living in Se-rich soils did not develop Keshan disease, although this was not the sole cause. This cardiomyopathy might be explained through a sequence of mechanisms presented in Fig. 1: Se deficiency leads to decreased GSH-Px and SOD activity and, consequently, to oxidative stress followed by a myocardial injury [54, 55].
Furthermore, some studies have shown a relationship between Se deficiency and heart diseases [54, 55]. Thus, Se-deficiency seems to be directly linked with at least one severe human illness; therefore, it appears prudent to avoid this deficiency [1, 52, 56]. This can be achieved by consumer awareness and seeking products that might complement this gap in regular food choices, like supplemented foods.
Lately, Se has been supplied to animals and humans through inorganic Se salt supplements [4]. However, these come with some drawbacks, including toxicity and poor absorption. Se-enriched supplements have been used to address or prevent diseases associated with Se deficiency-related affecting populations living in areas with low Se in soils [7, 54]. Biofortified foodstuffs, like Se-enriched plants, are commonly obtained through methods such as soil fertilization, foliar fertilization, and hydroponic fertilization [3]. Meat, dairy, and eggs can also be enriched with Se. A higher hen growth and Se content on Se-enriched eggs was achieved by feeding animals with Se-enriched insect protein, compared with Na2SeO3 or Se-yeast administration [3, 57]. Findings demonstrated that dietary supplementation with 2 mg Se kg−1 increased egg weight and improved its antioxidant capacity [3, 57]. Inorganic Se can also be incorporated to microorganism’s culture medium. Through interactions with proteins or polysaccharides, enriched Se foods such as yeast and fungi are produced [58] SeMet is the predominant dietary form of Se and is widely accepted as a supplement for both humans and animals [34]. Se-rich yeast used as a nutritional food additive is as safe as Se from other dietary sources, with the safety standards outlined in the Commission Implementing Regulation (EU) 2020/1993 of 4 December 2020 [7, 59].
For animal nutrition, several studies have reported that Se, at low levels, leads to deficient protection against oxidative stress, and consequently syndromes and diseases in animals [43, 54]. Various feeding techniques for different animals have been developed to elevate the Se concentration in animal products, with the goal of mitigating Se deficiency and potentially serve as a nutritional approach to manage diseases associated with free radicals [60].
Se has shown to protect against heavy metal poison, it plays a key role as a chelating agent for heavy metals by forming nontoxic Se-metal complexes. In animals exposed to the harmful effects of mercury (Hg), the Se compounds reduced the formation of necrotic lesions in kidneys [28]. Seafood with high Se levels could be a health benefit because Se levels could prevent oxidative damage related to Hg [61].
This micronutrient is frequently employed as a supplement to larvae to increase nutritional quality, enhancing antioxidant capacity and preventing diseases [5, 62], muscle atrophy, and skeletal deformations [5, 63]. The European Commission (EC) set upper threshold of 0.2 mg kg−1 Se-enriched yeast in aquaculture feeds ((EU) No 427/2013) [64].
Although Se deficiency is usually linked to poor nutrition, it could also be related to genetically disturbed Se-protein synthesis and abnormal transport of Se [31].
Se can cause depletion of the intracellular glutathione pool when consumed at high concentrations, resulting in ROS accumulation, which could induce cellular mortality [3, 29]. If the Se is not directly incorporated or bound by proteins, any excess in the organism is converted into methylated metabolites (like 1-β-methylseleno-N-acetyl-d galactosamine) and urea. H2Se, an intermediary product generated during various selenium compounds within cells, can be utilized for the synthesis of Se-proteins or undergo additional metabolism in the methylation pathway [31]. Given the narrow margin between Se deficiency and toxicity, in-depth research on organisms like microalgae is essential. Microalgae can serve as a primary source of Se for various organisms [4, 9, 29].
4 Selenium metabolism in microalgae
Microalgae have metabolic requirements for Se and therefore possess mechanisms for incorporating Se-AAs in essential Se-proteins. Thus, their capability to uptake and store Se varies based on the strain, growth conditions, and provided Se chemical form [4, 45]. The selection of the Se source is not clear, as for many microalgal species, selenate might present a greater toxicity risk compared to selenite, while the opposite may be true for others [7, 45].
Selenate exhibits high solubility, and thus more bioavailable to aquatic organisms than selenite, indicating that selenate may dissolve as the primary chemical species in an aqueous solution [7]. However, the interconversion of selenate and selenite is influenced by the chemical and physical characteristics of the water [2, 11, 65]. Studies on uptake suggest that microalgal cells can assimilate both selenite and selenate, influencing growth on a dose-dependent basis [4, 65].
The first algae in which Se-proteins were identified was Chlamydomonas reinhardtii and subsequently in other unicellular species [45, 66].
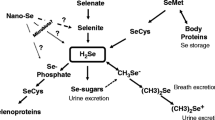
Microalgae exhibit efficient uptake of Se and convert it into less toxic organic forms (Se-AAs acids, Se-proteins, Se-PSs, and volatile compounds) [9]. Selenate, Se (VI), and selenite, Se (IV), are the primary forms of Se in aquatic environments, absorbed by microalgae [45] (Fig. 2, route 1). In general, Se (IV) is typically absorbed and accumulated more rapidly by microalgae when compared to Se (VI). This is because Se (IV) is carried through specific saturated transport system at low concentrations, and by a non-selective transport system at elevated concentrations (ATP-dependent) [9]. Upon internalization into microalgal cells, Se translocates to the chloroplasts and initiates the Se metabolic cascade, transforming into Se2−, the precursor for synthesizing SeCys and SeMet (Fig. 2, routes 1–4) [9, 45].
In aquatic environments where Se (VI) predominates as the primary form of available Se for uptake, it undergoes reduction to Se (IV) through a series of enzymatic reactions. Initially, adenosine triphosphate sulfurylase (ATPS) mediates the activation of Se (VI) by coupling it with ATP, resulting in the formation of adenosine phosphoselenate (APSe) [45]. The APSe is then further reduced to Se (IV) by APR (Fig. 2, route 2). However, the previous steps are unnecessary if Se (IV) is the primary substrate (Fig. 2, route 3) [9, 45].
Reduction of Se (IV) to Se2− can occur through enzymatic reactions involving the activity of sulphite reductase (SIR), or by non-enzymatic reactions in the presence of glutathione (GSH) (Fig. 2, route 4). SeCys can be directly synthesized from Se (IV) via the catalytic activity of selenomethyltransferase (SMT) (Fig. 2, route 5). SeCys serves as the initial substrate for synthesizing other seleno-compounds (Fig. 2, route 6) [45].
When the intracellular Se concentration exceeds a certain threshold, Se-AAs may lead to the production of aberrant proteins via non-specific incorporation. Thus, microalgae employ detoxification mechanisms to convert Se into volatile forms under such conditions. Specifically, SeCys is transformed into dimethyldiselenide (DMDSe) via the intermediate methylselenocysteine (MeSeCys-C4H9NO2Se) (Fig. 2, route 7), while SeMet undergoes methylation to yield DMSe [4] (Fig. 2, route 8). MeSeCys, also known as Se-methylselenocysteine is the result of SeCys methylation for accumulation [9]. Some microalgae, such as Chlorella sp., exhibit a high capacity to synthesise the Se volatile compounds DMDSe and/or DMSe upon selenate or selenite supply (Fig. 2, route 9); however further studies are required to elucidate this metabolic pathway [4, 29].
5 Microalgae enriched with selenium
Se content in terrestrial plants depends on the concentrations found in agricultural soils. Thus, Se plant content can be increased, at the expense of releasing it into the environment [3, 43]. In contrast, microalgae cultivation can be achieved in closed systems, and these organisms are much more efficient in capturing nutrients from the medium than terrestrial plants [16, 67]. Recently, there has been considerable interest in Se-enriched microalgae as a convenient method for generating readily accessible and bioavailable source of organically-bound Se [10, 11].
5.1 Selenium sources
5.1.1 Inorganic Se–Selenite and Selenate sodium salts
Both sodium selenate and selenite can be used as Se sources for enriching microalgae [68]. Sodium selenate (Na2SeO4) is a water-soluble compound, offering microalgae an easily accessible Se source. It is often used in Se-enrichment studies due to its stability and ease of handling. Sodium selenite (Na2SeO3) is another commonly used Se compound for microalgae enrichment. Like sodium selenate, sodium selenite is water-soluble and provides a source of bioavailable Se. Both are generally added to the microalgae culture medium in a controlled concentration. Sodium selenite is often preferred over sodium selenate due to its higher bioavailability [9, 68, 69], but the choice between both chemical compounds for microalgae enrichment depends on factors such as the specific microalgae species, growth conditions, and the desired Se content in the enriched microalgae biomass. Guimarães et al. [68] assessed the suitability of two Se species (Na2SeO3 and Na2SeO4) in producing Se-fortified Nannochloropsis oceanica biomass for aquafeed at various concentrations during a twelve-day cultivation period [68]. The values for 50% growth inhibition (EC50) for selenate (EC50 = 32.93 μM) had a more detrimental effect on microalgal growth than selenite (EC50 = 163.82 μM) [68].
The total intracellular Se content analysis revealed that N. oceanica exhibited a higher accumulation of selenite than selenate. Guimarães et al.’s [68] study summarises the inorganic Se sources applied to different microalgae species in different cultivation systems.
Se toxicity manifests as a decline in the exponential growth rate of microalgae [9]. Gojkovic et al. [38] noted that C. sorokiniana could adapt to selenate concentrations below 50 mg L−1. Their study revealed a 50% reduction in biomass concentration with the addition of 50 mg Se L−1 compared to the control. Additionally, Zhong and Cheng [33] illustrated a growth rate inhibition in C. pyrenoidosa at Na2SeO3 concentrations exceeding 40 mg L−1.
5.1.2 Se nanoparticles
Metal nanoparticles (NPs) have gained considerable interest owing to their remarkable characteristics, including high potential attributes, enhanced interfacial energy and ratio of surface area to volume, reduced fusion temperature, exceptional efficacy and specificity, quantum confinement effects, plasmonic resonance activation, and remarkable mechanical resilience [70,71,72]. The application of metallic nanoparticles covers various fields, namely related to environmental, agricultural, biomedical and electronics applications [73].
Given their nanoscale size and specific surface characteristics, metal NPs can easily penetrate cell walls and engage with internal biomolecules [74, 75]. Limited literature exists on studies investigating the impact of metal NPs on the development and metabolic output of microalgae. Some of these studies report that applying trace or low concentrations of NPs has been found to stimulate algal biomass and pigment content and enhance lipid production [73, 75,76,77]. Nevertheless, NPs biomedical applications are growing. Table 4 summarises potential health benefits of microalgae containing Se NPs [73].
Due to its redox properties, Se is biologically inactive and remains insoluble in water. However, Se nanoparticles (SeNPs) exhibit remarkable bioactivity and biosafety characteristics. SeNPs have gained significant attention due to their benefits, including the incorporation of Se in antioxidant defence systems and structure of numerous metabolic enzymes [35, 70, 78]. SeNPs have been acknowledged as a promising supplement to improve immune function and antioxidant potential [35, 71].
There are recent studies in which SeNPs were applied to the cultivation of Chlorella vulgaris [70, 73], but the authors' focus was not on the Se enrichment potential but on features such as the ability to promote the enhancement of algal biomass and lipids production, as well as the protective effect over oxidative stress generated by UV radiation. Further studies are needed on the evaluation of Se absorption by microalgae.
5.2 Se-enriched microalgae cultivation
Microalgae can be cultivated through three modes: autotrophic, heterotrophic, and mixotrophic.
-
Autotrophic cultivation: Requires light energy, an inorganic carbon source, water, and inorganic salts [26]. The photosynthetic microorganisms can be cultivated either in open (e.g., open raceway ponds) or closed systems (e.g., air-lift bioreactors, tubular photobioreactors, and flat-panel bioreactors) [26]. Most research on Se-enriched Chlorella focuses on optimizing small-scale phototrophic lab cultivation to maximize Se content, with fewer studies on large-scale production [4]. To the best of the authors’ knowledge, the study with the biggest scale-up cultivation for Se-enriched microalgae (2200 L) showed that cooler days with lower irradiation in photoautotrophic conditions can increase volatile Se compound production, although active growing cells reduce this [4].
-
Heterotrophic cultivation: Uses organic carbon such as glucose as a carbon and energy source, and grows without light, making it suitable for regions with insufficient sunlight [79]. Chlorella sp. grows faster and reaches higher cell densities under these conditions, tolerating higher Se concentrations. Se accumulation in biomass is proportional to Se dosage and increases linearly over time, similar to phototrophic cultures. The cultivation in a 9L column fermenter produced fewer volatile Se compounds compared to photoautotrophic cultivation in 2200 L raceway ponds [4].
-
Mixotrophic cultivation: Combines features of both auto- and heterotrophic methods, using light and organic carbon sources. This mode is underexplored, but shows potential for enriching Spirulina platensis. The lab-scale (250 mL Erlenmeyer’s growth) presented high Se-enriched biomass with the production of the important pigment, phycocyanin that is not available in the dark [80].
Each cultivation mode offers unique advantages for growth rates, Se enrichment, and production of specific compounds.
There are several strategies for improving biomass composition through modifications to the culture media [81]. Enriching microalgae with Se typically involve cultivating them in a controlled environment while supplying a Se source. It's worth mentioning that the specific protocol for Se enrichment of microalgae may vary depending on the species and the desired Se content. A general approach to enriching microalgae with Se usually comprises:
-
i)
The selection of microalgae species that are suitable for Se enrichment and have a high growth rate. Some widely studied microalgae species for Se enrichment include Chlorella sp., Spirulina sp., and Dunaliella salina;
-
ii)
Culture medium provides essential nutrients for microalgae growth, including a Se source. The specific composition of the culture medium will depend on the selected microalgae species. A medium with high levels of sulphur should be avoided because Se competes for the same cell transporters, and its availability decreases [4, 10]. Generally, it should contain macronutrients (nitrogen, phosphorus, potassium), micronutrients (iron, magnesium, sulphur, zinc, copper, sodium, etc.) [71], and a Se source such as sodium selenite or selenate.
-
iii)
The microalgae inoculation into the prepared culture medium [71];
-
iv)
Control over the environmental conditions;
-
v)
Regular microalgae growth monitoring, cell density, and Se content, as well as temperature, pH, light intensity, and photoperiod, shall be monitored. Optimal conditions vary depending on the microalgae species. To optimise growth and Se accumulation, the adjustment of the culture conditions, including nutrient concentrations and Se supplementation, may be necessary. Se toxicity can manifest as a reduction in the exponential growth rate of microalgae [4, 9], a yellowish color, and a garlic-like smell [4, 7];
-
vi)
Harvesting and processing of the enriched microalgae: once the microalgae have reached the desired Se enrichment level, they must be harvested. Depending on the intended application, the microalgae can be processed further, such as drying, cell disruption, or extraction, to obtain the Se-enriched biomass or extract [4, 69].
The action of Se salts on microalgae growth was tested in some species, especially Dunaliela salina and the genus Chlorella sp. [4]. Nevertheless, to the best of the authors' knowledge, a comparison between the trophic media and Se absorption was only done by Mylenko et al. [4] and Pires et al. [7].
The literature reports that Chlorella sp. tolerates higher selenite concentrations than selenate under both auto- and heterotrophic conditions [4, 6, 33]. In contrast to phototrophic growth, heterotrophic Chlorella sp. cultures exhibit greater tolerance to elevated Se concentrations [4]. Therefore, heterotrophic Chlorella sp. cultures offer an option to produce Se-enriched biomass with a higher content of organically bound Se [82]. Other studies have reported that some microalgae species could be exposed to high doses of Se at the exponential phase [69, 83].
SeMet was the main Se form bioaccumulated, followed by SeCys and MeSeCys in the phototrophic regime. The cumulative Se content within the biomass demonstrated a direct correlation with the Se dosage and exhibit linear growth over time. Notably, in heterotrophic cultivation, the concentrations of SeMet and SeCys were twice as abundant as those detected in the phototrophic cultures [4]. In the study by Pires et al. [7], higher Se absorption was obtained during autotrophic cultivation. The heterotrophic medium had higher phosphate and sulphate concentrations that competed for Se [7]. Se in the form of SeO32− inhibits membrane transporters for phosphates and suppresses the production of various phosphates and pyrophosphates essential for β-carotene synthesis [84]. Therefore, β-carotene levels could indirectly indicate the presence of Se in the medium [84].
According to Guimarães et al. [68], 30 μM of selenite in the growth medium led to Se bioaccumulation with minimal impact on cell growth. After 12 days, the intracellular Se content reached 0.131 g Se kg−1 biomass. Se application below 20 µM moderately boosted the photosynthetic activity and growth of C. vulgaris cultures. This was coupled with a notable Se assimilation into microalgae biomass (~ 0.5 mg Se g−1 DW) [85]. In the Goméz-Jacinto et al.'s [86] study, algal Se-enrichment of 3 µg g−1 was done within 100 h.
5.2.1 Impact of Se enrichment on the growth and metabolites production by microalgae
By examining the effects of Se supplementation on microalgae growth and chemical composition, we can pave the way for future research will explore the potential of microalgal biotechnology [9]. Microalgae Se enrichment is expected to influence the synthesis and accumulation of various metabolites in microalgae. For instance, Se-enrichment has been observed to enhance the production of different compounds such as proteins, fatty acids, carbohydrates, and carotenoids in certain microalgae species [37, 87,88,89].
Gojkovic et al. [88] explored how selenate influenced survival, cell structure, and SeMet accumulation in batch cultures of the green alga C. sorokiniana. The authors observed that comparing to the control, the supplementation with 40 mg Se L−1 decreased the growth rates by about 25% [90]. However, pigment levels were not affected by Se addition [74]. Gan et al. [91] have demonstrated that C. vulgaris can efficiently eliminate Se and generate saturated fatty acids, especially when Se is in the form of selenite. A higher percentage of saturated fatty acids, particularly palmitic acid, was noted in the sulphur (S) deprived algae exposed to either selenate or selenite. However, the highest lipid content (21.9%) was found in the algae treated with selenite [74].
Zheng et al. [34] investigated the effects of Se on both the growth rate and chlorophyll content of the unicellular green alga Haematococcus pluvialis. Their study found that elevated Se levels induced ROS formation, leading to the upregulation of antioxidant enzymes and increased production of astaxanthin. The findings shed new light on the toxic pathways of Se on microalgae, highlighting the correlation between tolerance to inorganic selenite, intracellular Se accumulation, the high ratio of organic Se, and astaxanthin content.
In Pires et al. [7], C. vulgaris was cultivated under two trophic regimes: autotrophic and heterotrophic. The cultivation media in both cases were supplemented with 20 mg L−1 sodium selenate. The biochemical composition of the Se-enriched biomass was comparable to the non-supplemented biomass, with slight differences observed in carbohydrate content (0.64% vs. 2.6%, respectively). The proportions of proteins (41% vs. 42%, respectively) and lipids (5.3% vs. 6.2%, respectively) were similar between the two conditions. However, variations were observed in the fatty acid profiles, with C18:1 and C18:0 being the major fatty acids present in Se-enriched microalgae. Similarly, different profiles of monosaccharides were observed, with glucose being the predominant monosaccharide on Se-enriched microalgae. The pigment content, including chlorophyll a, b, and total carotenoids, remained similar under both cultivation conditions relative to their non-enriched microalgae respective controls. Shangguan et al. [36] showed that C. pyrenoidosa supplementation with 20 mg L−1 sodium selenite improved not only the growth, but also the content of protein and soluble sugars, as well as antioxidant features, mainly SOD, GSH-Px and catalase.
Mylenko et al. [4] studied the assimilation of Se to amino acids in Chlorella sp. cultivated under both phototrophic and heterotrophic conditions. They examined the Se-AAs content, substitution rates of Se/S, and Se accumulation balance were examined in both growth modes of Chlorella sp. In large-scale experiments, both cultivation methods yielded comparable Se-AAs content. Outdoor phototrophic cultures achieved Se-AAs content of up to 400 μg g−1 and displayed a high Se/S substitution rate of 5–10%, with 30–60% of the organic/total Se incorporated into the biomass. Heterotrophic cultures in pilot-scale fermenters exhibited a higher Se-AAs content and Se/S substitution ratio. SeMet was the predominant form in both regimes: ∼ 275 μg g−1 DW and ∼ 430 μg g−1 DW for phototrophy and heterotrophy, respectively. Additionally, in phototrophic biomass, MeSeCys was the second most abundant, with ∼ 215 μg g−1 DW, while SeCys dominated in heterotrophic cultures, with ∼ 115 μg g−1 DW. In a recent study, C. vulgaris K-01 incorporated a maximum organic Se of 150 μg g−1 DW, with an EC50 value for the strain of 38.08 mg L−1 [92].
Se-polysaccharides derived from Se-enriched biomass refer to the combination of Se and polysaccharides obtained from biomass cultivated or modified to accumulate higher levels of Se. These polysaccharides serve as a carrier for Se, allowing for enhanced stability and bioavailability of the Se compound. Moreover, the combination of Se and polysaccharides provides a synergistic approach to harnessing the potential health benefits of both components: the antioxidant properties and Se role in various physiological processes in the human body and the diverse biological activities and health benefits of polysaccharides, including immune modulation, anti-inflammatory effects, and prebiotic properties [93, 94].
Natural Se polysaccharides are relatively rare and limited to a selected few plants and microorganisms capable of producing them in sufficient quantities [94]. Therefore, there is a need for research to focus on identifying Se-enriched polysaccharides and exploring methods to enhance the production of polysaccharides with higher Se content [94]. Currently, there are two primary approaches for synthetic Se polysaccharides. Firstly, one can enhance the Se content of polysaccharides within organisms by employing Se-enriched methods, such as subjecting them to stress in Se-enriched cultures. Alternatively, the Se content of polysaccharides can be elevated through selenation or chemical synthesis techniques [93].
Zhou et al.’s [30] study expanded the possibilities of utilizing S. platensis as a functional food source, revealing that the Se-containing polysaccharide effectively protected against cadmium-induced toxicity. This protective effect was superior to polysaccharide alone or sodium selenite alone [30]. Studies using enriched algal biomass are scarce, consequently, in-depth research both on the extraction processes and alternative chemical synthesis, as well as on structure, activity, pharmacological effects, structure–activity relationship, mechanisms, and clinical applications of Se polysaccharides holds significant value [30].
Whenever SeNPs were applied to microalgae cultivation, variations in the chemical composition of the algal biomass were also reported [70, 73]. Dinc et al. [70] confirmed that SeNPs under UV stress improved the antioxidant defence system in C. vulgaris via increased growth rate and biomass. In cultures containing SeNPs and UV-C exposed, the amount of H2O2 and malondialdehyde (oxidative stress biomarkers) decreased. The authors predicted that the performed study would facilitate a more effective extraction of valuable compounds like lipids and carotenoids. Nada et al. [73] demonstrated that biosynthesised NPs could effectively enhance microalgal biomass and lipid production, serving as a valuable resource for diverse applications. Lower concentrations of the synthesized NPs boosted both biomass and lipid output, with 50 mg L−1 of ZnONPs demonstrating the greatest capacity to improve both aspects. Saturated and polyunsaturated FA contents of C. vulgaris rose by 16% and 59%, respectively, while unsaturated FA content declined by 20% compared to the control [73].
Overall, these findings not only expand our knowledge of Se-microalgae interactions but also suggest that microalgae could become valuable resources with a broad spectrum of uses, including the development of feedstock and functional foods.
6 Se supplementation
6.1 Commercially available supplements
Commercial fertilisers may be a Se supplementation method that is too wasteful for widespread application due to environmental dispersion, while the food industry can directly add Se compounds into food products through fortification [3, 43, 95].
Some dietary supplements contain Se, for instance, SelenoPrecise and Se-ACE tablets. The former has l-selenomethionine, which is easily absorbed and utilised by the body, but no other seleno-amino acids. The latter combines vitamins A, C, and E [96]. Nevertheless, using multivitamins can be ineffective and counterproductive. For instance, vitamin B12 may degrade when exposed to vitamin C and copper, resulting in formation of inactive by-products [97]. l-selenomethionine has been approved as a Se source incorporated into food supplements for nutritional aims, at a dosage level under 250 µg day−1 for adults [98, 99].
A scientific opinion on the safety of selenite triglycerides as a source of Se included in food supplements, concluded that although the New Food (NF) is absorbed and supplies Se, the specific form in which it is absorbed remains unknown, and its bioavailability has not been determined yet [99]. The EFSA Panel on Nutrition, Novel Foods, and Food Allergens concluded that the NF's safety under the intended use conditions could not be established [99].
At present, Sel-Plex® (selenised yeast) is the sole product authorized as a nutritional feed additive in the EU [39, 100]. Under EU regulations (Regulation (EC) 1170/2009) governing the production of supplements containing Se, l-selenomethionine, and yeast enriched with Se can be employed in dietary supplements. Se-enriched yeast is produced by cultivating Saccharomyces cerevisiae supplemented with sodium selenate, as the source of Se, with its dry form containing no more than 2.5 mg Se g−1 [8, 101]. The main organic form of Se in yeast is SeMet (60–80% of total Se content in a preparation) [8, 59]. Greater bioaccessibility was found in Se-Chlorella sp. (∼ 49%) as compared to Se-yeast (∼ 21%), Se-supplement (∼3 2%), and Se-foods [10]. In fact, the bioaccessibility of Se-AAs from disintegrated spray-dried Se-Chlorella (~ 50%) was significantly higher than Se-yeast (~ 20%) and Se supplement (~ 30%; the Se form of the supplement is not described). The study suggests that processing Se-Chlorella to break down its rigid cell wall enhances its digestibility in the gastrointestinal tract. This is evident because Se-AAs from non-disintegrated Se-Chlorella are less bioaccessible compared to those from Se-yeast [10]. Another study showed that the bioavailability of Se increases when this element is supplemented by the algae instead of the basal diet of mice [90]. Furthermore, Castel et al. [102] study showed that supplementation of sodium selenite improved GSH-Px activities and selenoprotein expression, while a Se-enriched Spirulina was more effective in restoring selenium concentration, especially in the liver and kidney. Therefore, Se-microalgae could offer a great organic Se supplementation alternative. Few studies examine the impact of Se in both axenic cultures and co-cultures to enhance Se bioaccumulation, which could also serve as a method for Se-enrichment [103].
6.2 Microalgae biomass enriched with Se as a potential food and feed supplements
Se-enriched microalgae can be used across different industries and sectors, ranging from aquaculture and animal feed to human nutrition. Besides being able to convert inorganic into organic Se, microalgae present some advantages over the forms used for Se supplementation: the high content in EPA and DHA; the unique pigments (e.g., astaxanthin, β-carotene) and antioxidants, which have health benefits and commercial values. Besides, microalgae can convert CO2 and sunlight into biomass, making them more sustainable and environmentally friendly [16, 17]. Many works have shown that C. vulgaris is a suitable carrier for Se accumulation [4, 7, 9, 92, 104]. Table 5 summarises numerous studies conducted between 2010 and 2023 on the enrichment of microalgae with Se. These studies include microalgae species listed in the EU novel foods catalogue, as well as newly proposed algal species for introduction in the EU [16, 105]. Growth conditions, optimised Se concentrations, and Se supplementation in microalgal biomass are summarised. The table highlights numerous studies exploring Se supplementation in autotrophic microalgae across various cultivation systems, predominantly employing Erlenmeyer flasks. Most biomass was collected during the lag phase, and some studies proved that Se addition at the exponential phase might lead to higher Se accumulation in biomass [69, 90, 106]. Exposure duration and Se concentration varied, ranging from 0.5 to 50 mg.L−1 Na2SeO3 or Na2SeO4, with Na2SeO3 being the most frequently used compound. Due to its lower toxicity, Na2SeO3 has been used in a larger number of studies (Table 5). This might be the preferred salt for industrial use because it is a key ingredient in the formulation of Se-enriched yeast [13, 101].
From some studies, it is possible to conclude that inorganic Se was converted to organic species, and the most common form was SeMet [90].
The work of Mylenko et al. [4] demonstrated that, for Se daily requirements, humans would only need to consume 0.61 g day−1 of autotrophically Se-enriched C. vulgaris, which is a much smaller intake compared to other food products (7.2 g for broccoli, 1300 g for rice, and 300 g for potatoes) and with sodium selenite supplement (5.1 g) [7]. Se, primarily present as SeMet in C. sorokiniana, demonstrated favorable bioavailability in both in vitro and in vivo models [107]. Dolganyuk et al. [81] reported that a Se-chrome-lipid complex from C. vulgaris improved energy metabolism in rats and has the potential to regulate it in people with diabetes.
The evaluation of Se bioaccessibility in Se-enriched Chlorella sorokiniana, through in vitro gastrointestinal digestion of the selenised microalgae, revealed an 81% bioaccessibility (with 79% of Se present as SeMet). The bioavailability of Se, ranging from 3 to 15% depending on the diet, was 1.13-fold higher in mice fed with Se-enriched microalgae compared to those on a basal diet [90]. However, the authors determined that bioavailability decreased with higher levels of Se intake. The findings demonstrated that Se-enriched algae could be a viable option for selenised food for human consumption due to their high bioavailability of Se [90]. Other studies demonstrated that feed supplementation with Chlorella (0.5–1% of the diet) positively affected the growth performance of broilers [108] and, also thatadding small doses of Se-enriched Chlorella biomass to feedstock significantly enhanced the physiological characteristics of farmed animals [4, 109]. Kouba et al. [37] tested Se-enriched microalgae biomass in a fish diet, studying its storage in the muscle and liver, as well as different enzyme activities. Se concentrations suggested that Se from Se-enriched Chlorella sp. was more readily accumulated and exhibit greater biological activity, while being less toxic than sodium selenite [37]. Gilthead seabream larvae (Sparus aurata) supplemented with Se-fortified feed (11.65 mg kg−1 DW) showed enhanced survival rates and stress resilience [62]. Li et al. [110] explored the production of Se-enriched microalgae as a potential dietary supplement while simultaneously treating domestic wastewater. They concluded that inorganic Se absorbed by the microalgae was converted into Se-AAs (91%), and 49–63% of Se in the Se-enriched microalgae was bioavailable for animals [110]. In another research, in vivo studies on bioavailability, toxicity, and antioxidant defense of organic Se-enriched microalga biomass in Wistar rats were performed [111]. The study showed that organic Se-enriched Nannochloropsis oceanica CASA CC201 was not toxic to animals and significantly promoted their growth. It helped reduce cholesterol and low-density lipoprotein compared to the control. The organic Se-fed group had significant selenium accumulation in serum and tissues and lower oxidative stress marker malondialdehyde levels compared to the oxidative stress-induced group fed with a known antioxidant (silymarin). The study concluded that organic Se-enriched N. oceanica CASA CC201 could be an effective dietary selenium supplement [111].
Notably, these investigations demonstrate the efficacy of Se-enriched microalgae as a source of bioavailable Se, with SeMet being a common organic species detected. Se-enriched microalgae show potential as effective feed supplements for livestock and aquaculture, positively impacting growth performance and stress resistance in various species, also offering benefits for human consumption. Genetic engineering offers the potential to produce Se-enriched biomass. Nevertheless, when compared to bacteria, fungi, and yeast, this technique has not been explored [103]. There is still a lack of understanding of microalgae metabolism and regulation, as well as a lack of consistent transformation methods for a wide variety of species [112]. The Chlorophycae family exhibits limited gene consistency across different species, suggesting a highly variable capacity for environmental adaptation to Se. This diversity offers significant potential for genetic modifications and the development of new Se-based products. Three of the most mentioned genes SelD, SelB, and SEPSeCS are involved in the synthesis and targeted incorporation of SeCys into proteins [103]. Based on genetic engineering applied to other microorganisms, changes in the Se biosynthesis pathway could boost the production of a particular SeAA, also the upregulation of the mentioned genes could further promote the organic Se present in the cells [113].
7 Conclusions and future challenges
Applying Se-enriched microalgae as food and feed holds great promise and potential. Various research studies and experiments have established that microalgae can effectively accumulate and incorporate Se into their biomass in the more readily available organoselenium compounds. Nevertheless, its scalability, most possibly autotrophic, offers some challenges like optimizing growth conditions, and bioreactor design, but the main challenge is related to Se residues from harvesting. From the Se added into the culture media, only a part would be absorbed by microalgae, therefore some inorganic Se remains in the supernatant. This supernatant should undergo a treatment process to remove or neutralize the remaining inorganic residues to ensure they are not released into the environment. Alternatively, it can be used in another cycle of Se-enriched microalgae cultivation, maximizing the Se use. Larger production facilities could reduce the per-unit cost of Se-enriched microalgae, as well as the efficient use of nutrients. Utilizing microalgae for Se biofortification is environmentally friendly, as it can be produced with minimal land use and can help mitigate carbon emissions, which can enhance the product’s appeal in markets that prioritize eco-friendly solutions.
Se-enriched microalgae have the potential to address Se deficiencies in both humans and animals, offering numerous health benefits. Moreover, microalgae cultivation for Se enrichment can be environmentally sustainable, as it can be carried out using low-cost and readily available resources. Adding Se to microalgae enhances its nutritional value and economic potential as a nutritional supplement. Overall, applying Se-enriched microalgae as food and feed holds immense potential to address nutritional deficiencies, improve animal health, promote sustainable aquaculture, develop functional foods, and contribute to environmental sustainability.
Continued research and development in microalgae biotechnology can significantly advance Se-enriched microalgae production. This can include genetic engineering approaches to enhance Se accumulation, optimizing growth and supplementation conditions, and developing efficient harvesting and processing techniques. The chemical forms and concentrations of Se in microalgae biomass play a vital role in determining their biological properties. Understanding the accumulation and speciation of Se compounds in this biomass can offer valuable insights into the mechanisms behind the bioactivities of Se-enriched microalgae. These bioactivities encompass a range of beneficial effects, such as antioxidant and anticancer properties.
Future studies should contemplate extensive research activities to fully explore the potential of Se-enriched microalgae. These include determining the Se content and identifying its chemical forms, focusing on Se speciation in Se-enriched microalgae biomass. Additionally, conducting in vitro assays and in vivo animal studies to screen the biological effects of these enriched microalgae and validating the findings through human clinical trials will provide robust evidence and knowledge in this field, and facilitate its regulatory approval. Achieving regulatory acceptance for Se-enriched microalgae is relatively straightforward, particularly if the microalgae species used are already approved for food and feed applications, and the form of Se supplemented is also pre-approved, making it easier to meet regulatory requirements.
The culmination of such research efforts can motivate the food and feed industries to develop and produce Se-enriched microalgae biomass. By addressing Se deficiency and improving the quality of life for the global population, these nutritional products can significantly impact human and animal health and well-being.
Data availability
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
Abbreviations
- APR:
-
Adenosine phosphosulfate reductase
- ApSe:
-
Adenosine phosposelenate
- DHA:
-
Docosahexaenoic acid
- DMDSe:
-
Dimethyldiselenide
- DMSe:
-
Dimethylselenide
- EC:
-
European Commission
- EPA:
-
Eicosapentaenoic acid
- GSH-Px:
-
Glutathione peroxidase
- H2Se:
-
Hydrogen selenide
- MeSeCys:
-
Methylselenocysteine
- NF:
-
New food
- NPs:
-
Nanoparticles
- PUFAs:
-
Polyunsaturated fatty acids
- ROS:
-
Reactive oxygen species
- Se-AAs:
-
Selenium amino acids
- SeCys:
-
Selenocysteine
- SeCys2:
-
Selenocystine
- SeMet:
-
Selenomethionine
- SeNPs:
-
Selenium nanoparticles
- SeO2 :
-
Selenium oxide
- Se-PSs:
-
Selenium polysaccharides
- SIR:
-
Sulphite reductase
- SMT:
-
Selenomethyltransferase
- SOD:
-
Superoxide dismutase
References
Ye R, Huang J, Wang Z, Chen Y, Dong Y. The role and mechanism of essential selenoproteins for homeostasis. Antioxidants. 2022;11:973. https://doi.org/10.3390/antiox11050973.
Galić L, Galić V, Ivezić V, Zebec V, Jović J, Đikić M, Filipović A, Manojlović M, Almås ÅR, Lončarić Z. Modelling leverage of different soil properties on selenium water-solubility in soils of Southeast Europe. Agronomy. 2023;13:824. https://doi.org/10.3390/agronomy13030824.
Chen Z, Lu Y, Dun X, Wang X, Wang H. Research progress of selenium-enriched foods. Nutrients. 2023;15:4189. https://doi.org/10.3390/nu15194189.
Mylenko M, Vu DL, Kuta J, Ranglová K, Kubáč D, Lakatos G, Grivalský T, Caporgno MP, Da Câmara Manoel JA, Kopecký J, Masojídek J, Hrouzek P. Selenium incorporation to amino acids in Chlorella cultures grown in phototrophic and heterotrophic regimes. J Agric Food Chem. 2020;68:1654–65. https://doi.org/10.1021/acs.jafc.9b06196.
Samat NA, Yusoff FM, Rasdi NW, Karim M. Enhancement of live food nutritional status with essential nutrients for improving aquatic animal health: a review. Animals. 2020;10:1–27. https://doi.org/10.3390/ani10122457.
Zhang H, Zhao Z, Zhang X, Zhang W, Huang L, Zhang Z, Yuan L, Liu X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019;286:550–6. https://doi.org/10.1016/j.foodchem.2019.01.185.
Pires R, Costa M, Silva J, Pedras B, Concórdio-Reis P, Lapa N, Ventura M. Se-enrichment of Chlorella vulgaris grown under different trophic states for food supplementation. Algal Res. 2022;68: 102876. https://doi.org/10.1016/j.algal.2022.102876.
Niedzielski P, Rudnicka M, Wachelka M, Kozak L, Rzany M, Wozniak M, Kaskow Z. Selenium species in selenium fortified dietary supplements. Food Chem. 2016;190:454–9. https://doi.org/10.1016/j.foodchem.2015.05.125.
Gojkovic Ž, Garbayo I, Ariza JLG, Márová I, Vílchez C. Selenium bioaccumulation and toxicity in cultures of green microalgae. Algal Res. 2015;7:106–16. https://doi.org/10.1016/j.algal.2014.12.008.
Vu DL, Saurav K, Mylenko M, Ranglová K, Kuta J, Ewe D, Masojídek J, Hrouzek P. In vitro bioaccessibility of selenoamino acids from selenium (Se)-enriched Chlorella vulgaris biomass in comparison to selenized yeast; a Se-enriched food supplement; and Se-rich foods. Food Chem. 2019;279:12–9. https://doi.org/10.1016/j.foodchem.2018.12.004.
Schiavon M, Vecchia FD. Selenium and algae: accumulation, tolerance mechanisms and dietary perspectives. Selenium Plants: Mol Physiol Ecol Evolut Aspects. 2017. https://doi.org/10.1007/978-3-319-56249-0_5.
Pyett S, Jenkins W, Van Mierlo B, Trindade LM, Welch D, Van Zanten H. Our future protein, 2020. https://doi.org/10.1111/newe.12215
Zdziebłowska S, Zajda J, Ruzik L. Microalgae enriched in selenium as a good source of micronutrients. Food Biosci. 2024;59: 103908. https://doi.org/10.1016/j.fbio.2024.103908.
Yeiser M, Harris CL, Kirchoff AL, Patterson AC, Wampler JL, Zissman EN, Berseth CL. Growth and tolerance of infants fed formula with a new algal source of docosahexaenoic acid: double-blind, randomized, controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2016;115:89–96. https://doi.org/10.1016/j.plefa.2016.09.001.
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL. Microalgae: a potential alternative to health supplementation for humans. Food Sci Human Wellness. 2019;8:16–24. https://doi.org/10.1016/j.fshw.2019.03.001.
Mendes MC, Navalho S, Ferreira A, Paulino C, Figueiredo D, Silva D, Gao F, Gama F, Bombo G, Jacinto R, Aveiro SS, Schulze PSC, Gonçalves AT, Pereira H, Gouveia L, Patarra RF, Abreu MH, Silva JL, Navalho J, Varela JCS, Speranza LG. Algae as food in Europe: an overview of species diversity and their application†. Foods. 2022;11:1–36. https://doi.org/10.3390/foods11131871.
Ferreira de Oliveira AP, Bragotto APA. Microalgae-based products: food and public health. Future Foods. 2022;6: 100157. https://doi.org/10.1016/j.fufo.2022.100157.
Kotrbáček V, Doubek J, Doucha J. The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Phycol. 2015;27:2173–80. https://doi.org/10.1007/s10811-014-0516-y.
Rahim A, Çakir C, Ozturk M, Şahin B, Soulaimani A, Sibaoueih M, Nasser B, Eddoha R, Essamadi A, El Amiri B. Chemical characterization and nutritional value of Spirulina platensis cultivated in natural conditions of Chichaoua region (Morocco). S Afr J Bot. 2021;141:235–42. https://doi.org/10.1016/j.sajb.2021.05.006.
Cabrita ARJ, Guilherme-Fernandes J, Valente IM, Almeida A, Lima SAC, Fonseca AJM, Maia MRG. Nutritional composition and untargeted metabolomics reveal the potential of Tetradesmus obliquus, Chlorella vulgaris and Nannochloropsis oceanica as valuable nutrient sources for dogs. Animals. 2022;12:2643. https://doi.org/10.3390/ani12192643.
Muys M, Sui Y, Schwaiger B, Lesueur C, Vandenheuvel D, Vermeir P, Vlaeminck SE. High variability in nutritional value and safety of commercially available Chlorella and Spirulina biomass indicates the need for smart production strategies. Bioresour Technol. 2019;275:247–57. https://doi.org/10.1016/j.biortech.2018.12.059.
Zanella L, Vianello F. Microalgae of the genus Nannochloropsis: chemical composition and functional implications for human nutrition. J Funct Foods. 2020;68: 103919. https://doi.org/10.1016/j.jff.2020.103919.
Wan M, Jin X, Xia J, Rosenberg JN, Yu G, Nie Z, Oyler GA, Betenbaugh MJ. The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl Microbiol Biotechnol. 2014;98:9473–81. https://doi.org/10.1007/s00253-014-6088-6.
Gharibzahedi SMT, Jafari SM. The importance of minerals in human nutrition: bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci Technol. 2017;62:119–32. https://doi.org/10.1016/j.tifs.2017.02.017.
Bogstie C, Gallant M, Elphick JR, Kennedy C. The relationship between cellular protein content and selenium accumulation in freshwater microalgae. Integr Environ Assess Manag. 2024. https://doi.org/10.1002/ieam.4946.
Perez-Garcia O, Bashan Y. Algal Biorefineries. Cham: Springer International Publishing; 2015. p. 1–557. https://doi.org/10.1007/978-3-319-20200-6.
Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Poulsen M, Sanz Y, Schlatter JR, van Loveren H, Ferreira L, Knutsen HK. Safety of Schizochytrium sp. oil as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020. https://doi.org/10.2903/j.efsa.2020.6242.
Kieliszek M. Selenium–fascinating microelement, properties and sources in food. Molecules. 2019;24:1298. https://doi.org/10.3390/molecules24071298.
Schiavon M, Ertani A, Parrasia S, Vecchia FD. Selenium accumulation and metabolism in algae. Aquat Toxicol. 2017;189:1–8. https://doi.org/10.1016/j.aquatox.2017.05.011.
Zhou N, Long H, Wang C, Zhu Z, Yu L, Yang W, Ren X, Liu X. Characterization of selenium-containing polysaccharide from Spirulina platensis and its protective role against Cd-induced toxicity. Int J Biol Macromol. 2020;164:2465–76. https://doi.org/10.1016/j.ijbiomac.2020.08.100.
Kieliszek M, Błazejak S. Selenium: significance, and outlook for supplementation. Nutrition. 2013;29:713–8. https://doi.org/10.1016/j.nut.2012.11.012.
Kieliszek M, Błazejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21:609. https://doi.org/10.3390/molecules21050609.
Zhong Y, Cheng JJ. Effects of selenite on unicellular green microalga Chlorella pyrenoidosa: bioaccumulation of selenium, enhancement of photosynthetic pigments, and amino acid production. J Agric Food Chem. 2017;65:10875–83. https://doi.org/10.1021/acs.jafc.7b04246.
Zheng Y, Li Z, Tao M, Li J, Hu Z. Effects of selenite on green microalga Haematococcus pluvialis: bioaccumulation of selenium and enhancement of astaxanthin production. Aquat Toxicol. 2017;183:21–7. https://doi.org/10.1016/j.aquatox.2016.12.008.
Ferro C, Florindo HF, Santos HA. Selenium nanoparticles for biomedical applications: from development and characterization to therapeutics. Adv Health Mater. 2021;10:1–50. https://doi.org/10.1002/adhm.202100598.
Shangguan J, Yang N, Zhang L, Liu J, Li Y, Xu J, Xia X, Xu B. Sodium selenite enhances the production of functional proteins and biomass in Chlorella pyrenoidosa 038F by promoting acetate assimilation under heterotrophic cultivation. Aquaculture. 2024;589: 740987. https://doi.org/10.1016/j.aquaculture.2024.740987.
Kouba A, Velíšek J, Stará A, Masojídek J, Kozák P. Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel (Barbus barbus). Biomed Res Int. 2014. https://doi.org/10.1155/2014/408270.
Gojkovic Ž, Garbayo-Nores I, Gómez-Jacinto V, García-Barrera T, Gómez-Ariza JL, Márová I, Vílchez-Lobato C. Continuous production of selenomethionine-enriched Chlorella sorokiniana biomass in a photobioreactor. Process Biochem. 2013;48:1235–41. https://doi.org/10.1016/j.procbio.2013.06.013.
Doucha J, Lívanský K, Kotrbáček V, Zachleder V. Production of Chlorella biomass enriched by selenium and its use in animal nutrition: a review. Appl Microbiol Biotechnol. 2009;83:1001–8. https://doi.org/10.1007/s00253-009-2058-9.
Guimarães BO, Villarreal-Toribio B, García-Barrera T, Arias-Borrego A, Gremmen P, Wijffels RH, Barbosa MJ, D’Adamo S. Effect of sulphur on selenium accumulation and speciation in Nannochloropsis oceanica. J Funct Foods. 2022;96: 105215. https://doi.org/10.1016/j.jff.2022.105215.
Ullah H, Liu G, Yousaf B, Ali MU, Irshad S, Abbas Q, Ahmad R. A comprehensive review on environmental transformation of selenium: recent advances and research perspectives. Environ Geochem Health. 2019. https://doi.org/10.1007/s10653-018-0195-8.
Dobrzyńska M, Drzymała-Czyż S, Woźniak D, Drzymała S, Przysławski J. Natural sources of selenium as functional food products for chemoprevention. Foods. 2023;12:1247. https://doi.org/10.3390/foods12061247.
Alfthan G, Eurola M, Ekholm P, Venäläinen ER, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J Trace Elem Med Biol. 2015;31:142–7. https://doi.org/10.1016/j.jtemb.2014.04.009.
Ventura MG, Stibilj V, do Freitas MC, Pacheco AMG. Determination of ultratrace levels of selenium in fruit and vegetable samples grown and consumed in Portugal. Food Chem. 2009;115:200–6. https://doi.org/10.1016/j.foodchem.2008.10.089.
Ponton DE, Graves SD, Fortin C, Janz D, Amyot M, Schiavon M. Selenium Interactions with Algae: chemical processes at biological uptake sites, bioaccumulation, and intracellular metabolism. Plants. 2020;9:528. https://doi.org/10.3390/plants9040528.
Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Peláez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Aggett P, Crous Bou M, Cubadda F, Ciccolallo L, de Sesmaisons Lecarré A, Fabiani L, Titz A, Naska A. Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2023. https://doi.org/10.2903/j.efsa.2023.7704.
Henry MA, Fountoulaki E, Vasilaki A, Rigos G, Kokou F, Karalazos V. Dietary micronutrient supplementation in low fishmeal based diets for optimum growth and immune status of European sea bass (Dicentrarchus labrax) juveniles. Aquaculture. 2020;528: 735479. https://doi.org/10.1016/j.aquaculture.2020.735479.
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Donald Malevu T, Sochor J, Baron M, Melcova M, Zidkova J, Kizek R. A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int J Mol Sci. 2017;18:2209. https://doi.org/10.3390/ijms18102209.
Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci Total Environ. 2008;400:115–41. https://doi.org/10.1016/j.scitotenv.2008.06.024.
Ventura MG, de Freitas MC, Pacheco AMG. Selenium levels in mainland Portugal. Water Air Soil Pollut. 2005;166:167–79. https://doi.org/10.1007/s11270-005-7322-8.
Ye R, Huang J, Wang Z, Chen Y. Trace element selenium effectively alleviates intestinal diseases. Int J Mol Sci. 2021;22:11708.
Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, Bachmann M, Minich WB, Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12:1–13. https://doi.org/10.3390/nu12072098.
Fakhrolmobasheri M, Mazaheri-Tehrani S, Kieliszek M, Zeinalian M, Abbasi M, Karimi F, Mozafari AM. COVID-19 and selenium deficiency: a systematic review. Biol Trace Elem Res. 2022;200:3945–56. https://doi.org/10.1007/s12011-021-02997-4.
Sun Y, Wang Z, Gong P, Yao W, Ba Q, Wang H. Review on the health-promoting effect of adequate selenium status. Front Nutr. 2023. https://doi.org/10.3389/fnut.2023.1136458.
Loscalzo J. Basic implications of clinical observations- Keshan disease, selenium deficiency, and selenoproteome. Clin Transl Neurosci. 2014;2:1756–60. https://doi.org/10.1177/2514183X18789327.
Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68. https://doi.org/10.1016/S0140-6736(11)61452-9.
Qiu K, Zheng JJ, Obianwuna UE, Wang J, Zhang HJ, Qi GH, Wu SG. Effects of dietary selenium sources on physiological status of laying hens and production of selenium-enriched eggs. Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.726770.
Wu G, Liu F, Sun X, Lin X, Zhan F, Fu Z. Preparation of selenium-enriched yeast by re-using discarded Saccharomyces cerevisiae from the beer industry for se-supplemented fodder applications. Appl Sci. 2019. https://doi.org/10.3390/app9183777.
Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Poulsen M, Sanz Y, Schlatter JR, van Loveren H, Ackerl R, Knutsen HK. Safety of selenium-enriched biomass of Yarrowia lipolytica as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020;18:1–13. https://doi.org/10.2903/j.efsa.2020.5992.
Gu X, Gao C. New horizons for selenium in animal nutrition and functional foods. Anim Nutr. 2022;11:80–6. https://doi.org/10.1016/j.aninu.2022.06.013.
Ralston NVC. Selenium health benefit values as seafood safety criteria. EcoHealth. 2008;5:442–55. https://doi.org/10.1007/s10393-008-0202-0.
Saleh R, Betancor MB, Roo J, Montero D, Zamorano MJ, Izquierdo M. Selenium levels in early weaning diets for gilthead seabream larvae. Aquaculture. 2014;426–427:256–63. https://doi.org/10.1016/j.aquaculture.2014.02.011.
Fotedar R, Munilkumar S. Effects of organic selenium supplementation on growth, glutathione peroxidase activity and histopathology in juvenile barramundi (Lates calcarifer Bloch 1970) fed high lupin meal-based diets. Aquaculture. 2016;20(457):15–23.
Official Journal of the European Union, Commission Implementing Regulation (EU) No 427/2013 of 8 May 2013, 2012.
Schiavon M, Pilon-Smits EAH, Citta A, Folda A, Rigobello MP, Dalla Vecchia F. Comparative effects of selenate and selenite on selenium accumulation, morphophysiology, and glutathione synthesis in Ulva australis. Environ Sci Pollut Res. 2016;23:15023–32. https://doi.org/10.1007/s11356-016-6649-6.
Chen N, Zhao C, Zhang T. Selenium transformation and selenium-rich foods. Food Biosci. 2021;40: 100875. https://doi.org/10.1016/j.fbio.2020.100875.
Prokop A, Bajpai RK, Zappi ME. Algal biorefineries: Volume 2: Products and refinery design, 2015. https://doi.org/10.1007/978-3-319-20200-6.
Guimarães BO, de Boer K, Gremmen P, Drinkwaard A, Wieggers R, Wijffels RH, Barbosa MJ, D’Adamo S. Selenium enrichment in the marine microalga Nannochloropsis oceanica. Algal Res. 2021;59: 102427. https://doi.org/10.1016/j.algal.2021.102427.
Zhao Y, Song X, Cao X, Wang Y, Si Z, Chen Y. Toxic effect and bioaccumulation of selenium in green alga Chlorella pyrenoidosa. J Appl Phycol. 2019;31:1733–42. https://doi.org/10.1007/s10811-018-1711-z.
Dinc SK, Vural OA, Kayhan FE, San Keskin NO. Facile biogenic selenium nanoparticle synthesis, characterization and effects on oxidative stress generated by UV in microalgae. Particuology. 2022;70:30–42. https://doi.org/10.1016/j.partic.2021.12.005.
Menon S, Shrudhi Devi KS, Santhiya R, Rajeshkumar S, Venkat Kumar S. Selenium nanoparticles: a potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf B Biointerfaces. 2018;170:280–92. https://doi.org/10.1016/j.colsurfb.2018.06.006.
Singh RD, Sethy S, Ghosh S, Srivastava AK. UV and γ-radiation induced molecular changes for rapid lipid accumulation in Chlorella sorokiniana. Biomass Bioenergy. 2022;163: 106493. https://doi.org/10.1016/j.biombioe.2022.106493.
Nada HG, Ali HEA, El-Behery RR, Shanab SMM, Elshatoury EH. Nanoparticles biosynthesized by Bacillus cereus filtrate and gamma rays enhancing Chlorella vulgaris biomass and lipid production. J Clust Sci. 2022;33:2055–68. https://doi.org/10.1007/s10876-021-02122-4.
AbdulRazack S, Duraiarasan S, Mani V. Biosynthesis of silver nanoparticle and its application in cell wall disruption to release carbohydrate and lipid from C. vulgaris for biofuel production. Biotechnol Rep. 2016;11:70–6. https://doi.org/10.1016/j.btre.2016.07.001.
Shanab SMM, Partila AM, Ali HEA, Abdullah MA. Impact of gamma-irradiated silver nanoparticles biosynthesized from Pseudomonas aeruginosa on growth, lipid, and carbohydrates of Chlorella vulgaris and Dictyochloropsis splendida. J Radiat Res Appl Sci. 2021;14:70–81. https://doi.org/10.1080/16878507.2020.1856599.
BehzadiTayemeh M, Esmailbeigi M, Shirdel I, Joo HS, Johari SA, Banan A, Nourani H, Mashhadi H, Jami MJ, Tabarrok M. Perturbation of fatty acid composition, pigments, and growth indices of Chlorella vulgaris in response to silver ions and nanoparticles: a new holistic understanding of hidden ecotoxicological aspect of pollutants. Chemosphere. 2020;238: 124576. https://doi.org/10.1016/j.chemosphere.2019.124576.
Kang NK, Lee B, Choi GG, Moon M, Park MS, Lim JK, Yang JW. Enhancing lipid productivity of Chlorella vulgaris using oxidative stress by TiO2 nanoparticles. Korean J Chem Eng. 2014;31:861–7. https://doi.org/10.1007/s11814-013-0258-6.
Sarkar RD, Singh HB, Kalita MC. Enhanced lipid accumulation in microalgae through nanoparticle-mediated approach, for biodiesel production: a mini-review. Heliyon. 2021;7: e08057. https://doi.org/10.1016/j.heliyon.2021.e08057.
Barros A, Pereira H, Campos J, Marques A, Varela J, Silva J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci Rep. 2019;9:1–7. https://doi.org/10.1038/s41598-019-50206-z.
Chen T, Zheng W, Wong YS, Yang F, Bai Y. Accumulation of selenium in mixotrophic culture of Spirulina platensis on glucose. Bioresour Technol. 2006;97:2260–5. https://doi.org/10.1016/j.biortech.2005.10.038.
Dolganyuk V, Belova D, Babich O, Prosekov A, Ivanova S, Katserov D, Patyukov N, Sukhikh S. Microalgae: a promising source of valuable bioproducts. Biomolecules. 2020;10:1–24. https://doi.org/10.3390/biom10081153.
de Carvalho Silvello MA, Severo Gonçalves I, Patrícia Held Azambuja S, Silva Costa S, Garcia Pereira Silva P, Oliveira Santos L, Goldbeck R. Microalgae-based carbohydrates: a green innovative source of bioenergy. Bioresour Technol. 2022;344: 126304. https://doi.org/10.1016/j.biortech.2021.126304.
Perečinec MG, Babić S, Čižmek L, Selmani A, Popović NT, Sikirić MD, Strunjak-Perović I, Čož-Rakovac R. Selenite as a lipid inductor in marine microalga Dunaliella tertiolecta: comparison of one-stage and two-stage cultivation strategies. Appl Biochem Biotechnol. 2022;194:930–49. https://doi.org/10.1007/s12010-021-03659-w.
Kizovský M, Pilát Z, Mylenko M, Hrouzek P, Kuta J, Skoupý R, Krzyžánek V, Hrubanová K, Adamczyk O, Ježek J, Bernatová S, Klementová T, Gjevik A, Šiler M, Samek O, Zemánek P. Raman microspectroscopic analysis of selenium bioaccumulation by green alga Chlorella vulgaris. Biosensors. 2021;11:115. https://doi.org/10.3390/bios11040115.
Babaei A, Ranglová K, Malapascua JR, Masojídek J. The synergistic effect of Selenium (selenite, –SeO32−) dose and irradiance intensity in Chlorella cultures. AMB Express. 2017. https://doi.org/10.1186/s13568-017-0348-7.
Gómez-Jacinto V, García-Barrera T, Garbayo-Nores I, Vilchez-Lobato C, Gómez-Ariza JL. Metal-metabolomics of microalga Chlorella sorokiniana growing in selenium- and iodine-enriched media. Chem Pap. 2012;66:821–8. https://doi.org/10.2478/s11696-012-0186-7.
Barbosa MJ, Wijffels RH, Janssen M, Südfeld C, Adamo SD. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023;2289:1–20. https://doi.org/10.1016/j.tibtech.2022.12.017.
Gojkovic Ž, Vílchez C, Torronteras R, Vigara J, Gómez-Jacinto V, Janzer N, Gómez-Ariza JL, Márová I, Garbayo I. Effect of selenate on viability and selenomethionine accumulation of Chlorella sorokiniana grown in batch culture. Sci World J. 2014. https://doi.org/10.1155/2014/401265.
Jiru M, Stranska-Zachariasova M, Kohoutkova J, Schulzova V, Krmela A, Revenco D, Koplik R, Kastanek P, Fulin T, Hajslova J. Potential of microalgae as source of health-beneficial bioactive components in produced eggs. J Food Sci Technol. 2021. https://doi.org/10.1007/s13197-020-04896-3.
Gómez-Jacinto V, Navarro-Roldán F, Garbayo-Nores I, Vílchez-Lobato C, Borrego AA, García-Barrera T. In vitro selenium bioaccessibility combined with in vivo bioavailability and bioactivity in Se-enriched microalga (Chlorella sorokiniana) to be used as functional food. J Funct Foods. 2020;66: 103817. https://doi.org/10.1016/j.jff.2020.103817.
Gan X, Huang JC, Zhou C, He S, Zhou W. Relationship between selenium removal efficiency and production of lipid and hydrogen by Chlorella vulgaris. Chemosphere. 2019;217:825–32. https://doi.org/10.1016/j.chemosphere.2018.11.075.
Zhang Z, Zhang Y, Hua Y, Chen G, Fu P, Liu J. Heterotrophic selenium incorporation into Chlorella vulgaris K-01: selenium tolerance assimilation, and removal through microalgal cells. Foods. 2024;13:405. https://doi.org/10.3390/foods13030405.
Yang W, Huang G, Chen F, Huang H. Extraction/synthesis and biological activities of selenopolysaccharide. Trends Food Sci Technol. 2021;109:211–8. https://doi.org/10.1016/j.tifs.2021.01.028.
Li J, Shen B, Nie S, Duan Z, Chen K. A combination of selenium and polysaccharides: promising therapeutic potential. Carbohydr Polym. 2019;206:163–73. https://doi.org/10.1016/j.carbpol.2018.10.088.
Haug A, Graham RD, Christophersen OA, Lyons GH. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb Ecol Health Dis. 2007;19:209–28. https://doi.org/10.1080/08910600701698986.
Lavu RVS, Van De Wiele T, Pratti VL, Tack F, Du Laing G. Selenium bioaccessibility in stomach, small intestine and colon: comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem. 2016;197:382–7. https://doi.org/10.1016/j.foodchem.2015.08.001.
Pereira J, Simões M, Silva JL. Microalgal assimilation of vitamin B12 toward the production of a superfood. J Food Biochem. 2019;43:1–15. https://doi.org/10.1111/jfbc.12911.
Aguilar F, Charrondiere UR, Dusemund B, Galtier P, Gilbert J, Gott DM, Grilli S, Guertler R, Kass GEN, Koenig J, Lambré C, Larsen J-C, Leblanc J-C, Mortensen A, Parent-Massin D, Pratt I, Rietjens IMCM, Stankovic I, Tobback P, Verguieva T, Woutersen R. Scientific opinio L-selenomethionine as a source of selenium added for nutritional purposes to food supplements. EFSA J. 2009;1082:1–39. https://doi.org/10.2903/j.efsa.2009.1082.
Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Poulsen M, Schlatter JR, van Loveren H, Germini A, Knutsen HK. Scientific opinion on the safety of selenite triglycerides as a source of selenium added for nutritional purposes to food supplements. EFSA J. 2020. https://doi.org/10.2903/j.efsa.2020.6134.
EFSA Panel. Scientific opinion on safety and efficacy of Sel-Plex® (organic form of selenium produced by Saccharomyces cerevisiae CNCM I-3060) for all species. EFSA J. 2011;9:2110. https://doi.org/10.2903/j.efsa.2011.2110.
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kot AM. Effect of selenium on growth and antioxidative system of yeast cells. Mol Biol Rep. 2019;46:1797–808. https://doi.org/10.1007/s11033-019-04630-z.
Castel T, Léon K, Gandubert C, Gueguen B, Amérand A, Guernec A, Théron M, Pichavant-Rafini K. Comparison of sodium selenite and selenium-enriched Spirulina supplementation effects after selenium deficiency on growth, tissue selenium concentrations, antioxidant activities, and selenoprotein expression in rats. Biol Trace Elem Res. 2023. https://doi.org/10.1007/s12011-023-03705-0.
Hoyos BS, Hernandez-Tenorio F, Miranda AM, Villanueva-Mejía DF, Sáez AA. Systematic analysis of genes related to selenium bioaccumulation in microalgae: a review. Biology. 2023;12:703. https://doi.org/10.3390/biology12050703.
Sun X, Zhong Y, Huang Z, Yang Y. Selenium accumulation in unicellular green alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS ONE. 2014;9:1–8. https://doi.org/10.1371/journal.pone.0112270.
EC, Commission implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, Official Journal of the European Union 351 (2017) 1–188. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2470&from=EN.
Singh P, Singh S, Maurya P, Mohanta A, Dubey H, Khadim SR, Singh AK, Pandey AK, Singh AK, Asthana RK. Bioaccumulation of selenium in halotolerant microalga Dunaliella salina and its impact on photosynthesis, reactive oxygen species, antioxidative enzymes, and neutral lipids. Mar Pollut Bull. 2023;190: 114842. https://doi.org/10.1016/j.marpolbul.2023.114842.
Sandgruber F, Gielsdorf A, Baur AC, Schenz B, Müller SM, Schwerdtle T, Stangl GI, Griehl C, Lorkowski S, Dawczynski C. Variability in macro-and micronutrients of 15 commercially available microalgae powders. Mar Drugs. 2021;19:1–23. https://doi.org/10.3390/md19060310.
Abdelnour SA, Abd El-Hack ME, Arif M, Khafaga AF, Taha AE. The application of the microalgae Chlorella spp. As a supplement in broiler feed. Worlds Poult Sci J. 2019;75:305–18. https://doi.org/10.1017/S0043933919000047.
Costa MM, Spínola MP, Prates JAM. Microalgae as an alternative mineral source in poultry nutrition. Vet Sci. 2024;11:44. https://doi.org/10.3390/vetsci11010044.
Li J, Otero-Gonzalez L, Michiels J, Lens PNL, Du Laing G, Ferrer I. Production of selenium-enriched microalgae as potential feed supplement in high-rate algae ponds treating domestic wastewater. Bioresour Technol. 2021;333: 125239. https://doi.org/10.1016/j.biortech.2021.125239.
Ragini R, Arumugam M. In vivo studies on bioavailability, toxicity, and antioxidant defense of organic selenium-enriched microalga biomass in Wistar rats. J Appl Phycol. 2023;35:1699–713. https://doi.org/10.1007/s10811-023-03007-x.
Enzing C, Ploeg M, Barbosa M, Sijtsma L. Microalgae-based products for the food and feed sector: an outlook for. Europe. 2014. https://doi.org/10.2791/3339.
Nawaz T, Gu L, Fahad S, Saud S, Harrison MT, Zhou R. Sustainable protein production through genetic engineering of cyanobacteria and use of atmospheric N2 gas. Food Energy Secur. 2024. https://doi.org/10.1002/fes3.536.
de Morais EG, Murillo AM, Lens PNL, Ferrer I, Uggetti E. Selenium recovery from wastewater by the green microalgae Chlorella vulgaris and Scenedesmus sp. Sci Total Environ. 2022;851: 158337. https://doi.org/10.1016/j.scitotenv.2022.158337.
Ghaderpour S, Ahmadifard N, Agh N, Vahabzadeh Z, Estevez A. Short-term enrichment of microalgae with inorganic selenium and zinc and their effects on the mineral composition of microalgae and marine rotifer Brachionus plicatilis. Aquac Nutr. 2021;27:2772–85. https://doi.org/10.1111/anu.13406.
Reshma R, Arumugam M. Organic selenium fortification in edible marine microalga Nannochloropsis oceanica CASA CC201 for food and feed applications. Algal Res. 2022;66: 102787. https://doi.org/10.1016/j.algal.2022.102787.
Skřivan M, Skřivanová V, Dlouhá G, Brányiková I, Zachleder V, Vítová M. The use of selenium-enriched alga Scenedesmus quadricauda in a chicken diet. Czech J Anim Sci. 2010;55:565–71. https://doi.org/10.17221/2480-cjas.
Acknowledgements
This work received support and help from FCT/MCTES (LA/P/0008/2020 doi: https://doi.org/10.54499/LA/P/0008/2020, UIDP/50006/2020 doi: https://doi.org/10.54499/UIDP/50006/2020 and UIDB/50006/2020 doi: https://doi.org/10.54499/UIDB/50006/2020), through national funds. Rita Pires acknowledges the Ph.D. Fellowship 2022.12360.BNA, Ref CRM:001 1392 from Fundação para a Ciência e Tecnologia and Márcia Ventura thank the contract through program DL 57/2016 – Norma transitória.
Funding
This work received financial support from FCT/MCTES (UIDP/50006/2020 doi: https://doi.org/10.54499/UIDP/50006/2020) through national funds.
Author information
Authors and Affiliations
Contributions
M.V., N.L. and R.P. had the idea for the article, literature search and data analysis was made by R.P., M.V. and M.C. The first draft was written by R.P., M.V. and M.C. N.L., L.F., H.P., H.C. and J.S. critically revised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pires, R., Costa, M., Pereira, H. et al. Microalgae as a selenium vehicle for nutrition: a review. Discov Food 4, 84 (2024). https://doi.org/10.1007/s44187-024-00157-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00157-w