Abstract
Purpose
The WHO’s classification of renal cell carcinoma (RCC) has transitioned from solely morphologic to an integrated molecular-defined classification with genotyping based on driver genes. One-third of patients with localized diseases eventually develop metastases. First-line treatments with tyrosine kinase inhibitors and immunotherapies have shown promise in improving patient outcomes. This study aims to assess the efficacy of next-generation sequencing (NGS) guided therapy for patients with metastatic renal cell carcinoma (mRCC).
Methods
We evaluated a cohort of 77 mRCC patients. In the context of first-line systemic treatment, 35 patients were administered NGS-guided personalized therapy, while 42 received empirical therapy. We subsequently compared the progression-free survival (PFS) of both groups and reported the objective response rate (ORR) for the NGS-guided group.
Results
Over the past three years, the application rate of NGS post-nephrectomy in West China Hospital averaged 21%. The NGS-guided group showed a significantly better PFS (P = .01) compared to the empirical therapy group. The NGS-guided group also exhibited an ORR of 41.9% and a disease control rate (DCR) of 90.3%.
Conclusions
Our data highlights NGS as a crucial, clinically accessible genotyping method suitable for broader adoption. This study underscores that NGS-guided therapy can significantly enhance outcomes for mRCC patients, particularly for specific driving mutations, such as FH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Kidney cancer ranks as the 14th most prevalent malignancy worldwide, accounting for approximately 431,288 new diagnoses and 179,368 deaths in 2020 [1]. The majority of these cases are classified as Renal cell carcinoma (RCC). Historically, the subtypes of kidney cancer were determined based on their distinct morphological features. For instance, clear cell renal cell carcinomas (ccRCC) constitute about 70% of all kidney cancers, while papillary RCC and chromophobe RCC represent around 5% [2,3,4]. Despite this morphological categorization, RCCs exhibit considerable molecular heterogeneity. Therefore, the 2022 WHO classification incorporated a molecular-driven RCC classification alongside the traditional morphology-based system [5]. Current molecular classifications encompass TFE3-rearranged RCC, TFEB-altered RCC, ELOC-mutated RCC, FH-deficient RCC, ALK-rearranged RCC, SMARCB1-deficient medullary RCC, and SDH–deficient RCC. Due to limited testing technology in the past, the histopathological subtypes of RCC defined by molecules can be difficult to differentiate, resulting in incorrect diagnoses and subpar treatment outcomes. As research progresses, it’s anticipated that more subtypes with unique molecular backgrounds will be identified. For precise diagnosis and the formulation of individualized therapeutic strategies, it is imperative for urologists and pathologists to synergize next-generation sequencing with immunohistochemistry.
Although surgery and localized therapies play a pivotal role, the aggressive nature of RCC means that 30% of patients with localized diseases eventually develop metastases [6]. The last decade has witnessed a dynamic shift in the therapeutic landscape of metastatic RCC. In the early 2000s, Bevacizumab, the first anti-angiogenic agent, gained approval by targeting and neutralizing VEGF-A proteins. Subsequent years saw the emergence of multiple VEGFR inhibitors such as sorafenib, sunitinib, pazopanib, axitinib, lenvatinib, and cabozantinib, collectively known as tyrosine kinase inhibitors(TKIs) [7]. Another therapeutic option is the mammalian target of rapamycin (mTOR) inhibitors like everolimus, especially for patients with PI3K pathway mutations [8]. In recent years, novel immunotherapies(IOs) have been explored, such as the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)checkpoint inhibitors. KEYNOTE-426, CheckMate 9ER, CLEAR, and JAVELIN Renal 101 demonstrated benefits with doublet combinations of TKI and IO across multiple endpoints [9]. Further on, promising new agents are emerging, such as hypoxia-inducible factor inhibitor belzutifan. The refined process of treatment selection will hinge on analyzing genomic signatures and identifying biomarkers to ensure precise, patient-specific care.
The advent of next-generation sequencing (NGS) has revolutionized personalized medical care opportunities. In oncology, NGS sheds light on the intricate molecular changes, paving the way for treatments tailored to individual cancer characteristics. In this context, we delved into the application of NGS post-nephrectomy in our institution. Our analysis encompassed a cohort of 77 RCC patients, with 35 of them undergoing NGS profiling. This study underscores the transformative potential of NGS-driven personalized medical treatments.
2 Materials and methods
2.1 Patient population
We calculated the ratio of NGS to nephrectomy from 2017 to 2021. We conducted a single-center, retrospective review of patients with RCC who received NGS from September 2018 through December 2021 at West China Hospital. Patients were included according to the following eligibility criteria: patients were ≥ 18 years of age and pathologically diagnosed with RCC; confirmed distal metastasis by pathology or radiological imaging; Patients who were also diagnosed with other malignant diseases or major diseases were excluded from the analysis. Age, gender, International Metastatic RCC Database Consortium (IMDC) risk score at the start of therapy, sites of disease, TNM staging, ISUP grading, and first-line treatment regime were recorded.
2.2 Treatment and survival outcomes
Patients received either NGS-guided therapy or empirical therapy. Detailed therapeutic regimens include TKIs (sunitinib, axitinib et al.) alone or TKIs combined with IOs (axitinib plus sintilimab et al.) Tumors were assessed using computed tomography or MRI before the first-line therapy (baseline) and followed every 1 to 3 months until disease progression or discontinuation of treatment. The treatment responses of patients treated with TKIs were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria. Immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) was used to assess the treatment response of IO-treated patients. According to these criteria, treatment response was classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was defined as the percentage of patients with a confirmed CR or PR among all treated patients, while the disease control rate (DCR) was defined as the percentage of patients with confirmed CR, PR, or SD among all treated patients. The clinical endpoints were PFS1. PFS1 was considered as the time from the first administration to disease progression, death, or end of follow-up during first-line treatment.
2.3 DNA extraction
Concerning the isolation of genomic DNA, formalin-fixed, paraffin-embedded EC samples of 10 μm thickness were selected and deparaffinized. Subsequently, genomic DNA of tumor tissues was obtained with QIAamp DNA FFPE Tissue Kit consistent with the manufacturer’s protocols. The quantification of DNA and the evaluation of DNA quality were conducted on Qubit Fluorometer using a Qubit dsDNA HS Assay kit (Thermo Fisher) and Nanodrop 2000, respectively.
2.4 NGS library preparation and sequencing
A bioruptor instrument was utilized to shear DNA into 300 to 350 base pairs. A sequencing library was subsequently on-beads amplified based on The KAPA Hyper Prep Kit(Kapa Biosystems). Regarding hybridization capture enrichment, a cancer-related genes panel was added. The capture reaction of probe-binding fragments was carried out by Dynabeads M-270, and an enriched library was amplified using Kapa HiFi HotStart ReadyMix.
2.5 Statistical analysis
Statistical analysis was performed with R version 4.2.1 software. All tests were two-tailed, and p < 0.05 was considered statistically significant. PFS was calculated from the date of first administration to disease progression, death, or end of follow-up. The Kaplan–Meier method with a log-rank test was performed to determine whether the difference in survival was significant.
3 Result
3.1 The feasibility of implementing NGS in postoperation
Patients diagnosed with RCC at our center routinely undergo nephrectomy, which encompasses partial, radical, and cytoreductive nephrectomies, after thorough presurgical evaluations. Over the last five years, the number of these operations has remained relatively stable: 525 cases in 2017, 551 cases in 2018, 571 cases in 2019, 573 cases in 2020, and 621 cases in 2021. At each follow-up visit, the majority of these patients are assessed, and if deemed necessary, they receive adjuvant therapy, such as sunitinib.
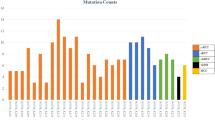
On the other hand, in the setting of metastatic renal cell carcinoma, urologists have increasingly recommended next-generation sequencing (NGS) in recent years. NGS primarily aids in the systematic selection of therapeutic regimens. To date, the first batch of samples was acquired in 2018, respectively from 16 patients. The subsequent years witnessed a rapid increase in participants in the NGS program: 98 cases in 2019 and 157 in 2020, stabilizing to 123 in 2021. Herein, we calculated the annual ratio of NGS participants to nephrectomy cases, yielding rates of 0%, 2.9%, 17.2%, 27.2%, and 19.8% for each successive year (Table 1).
3.2 Clinical characterization
Table 2 summarizes the basic information of the 77 RCC patients. Males constituted nearly 64.5% of the cases. The median age at the time of initial diagnosis stood at 44 years, with a range of 20 to 76 years. Notably, 62.7% of the patients were aged above 40 years. When classified based on the American Joint Committee on Cancer (AJCC) stage, 57.6% of patients were categorized as T3a or higher. According to the prevailing WHO/ISUP grading system, 45.7% of the tumor samples were graded at a score of 3. Regarding metastatic status, 45.6% of patients were confirmed to have two or more metastatic sites. The lymph node emerged as the most common metastatic site at 40.0%, closely followed by the lung at 33.8%. At the onset of first-line treatment, patients were categorized into International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) risk groups as follows: 12.5% in the favorable-risk group, 70.8% in the intermediate-risk group, and 16.7% in the poor-risk group. For the chosen first-line therapeutic regimen, the percentage of patients who received a combination of tyrosine kinase inhibitors and immunotherapy (51.9%) was nearly the same as those who were administered tyrosine kinase inhibitors alone (48.1%).
3.3 Clinical benefit of NGS-guided treatments
The NGS-guided group included 3 cases of complete response,10 of partial response, 15 of stable disease, and 3 of progression of disease with an objective remission rate of 41.9% and disease control rate of 90.3%. We conducted a survival analysis between NGS-guided therapy and the empirical therapy group. As expected, the NGS-guided therapy demonstrated a significant improvement in first-line progression-free survival (P = .01; Fig. 1). In essence, NGS data successfully informed the RCC treatment approach, whether through a combination of TKI and immunotherapy or a personalized TKI regimen alone.
4 Discussion
Our primary objective was to assess the role of next-generation sequencing (NGS) in the management of individuals with renal cell carcinoma (RCC). In this study, we demonstrated that it is feasible to incorporate NGS into routine RCC management, and the percentage of NGS was, on average, up to 21% over the last three years. Notably, in the NGS group, the non-clear cell RCC (nccRCC) subtype predominated, accounting for 54% in 2019, 71% in 2020, and 60% in 2021. Of the 35 individuals in our study, 24 underwent radical nephrectomy, while eight had previously undergone cytoreductive nephrectomy. Crucially, our data revealed that treatments tailored based on genomic insights significantly outperformed traditional empirical approaches regarding first-line progression-free survival, with 80% of patients attaining stable disease or better outcomes.
NGS is progressively adopted in clinical oncology due to its superiority over traditional sequencing. NGS allows simultaneous assessment of all druggable mutations, detection of high-level microsatellite instability(MSI-H), and evaluation of tumor mutation burden(TMB) and homologous recombination deficiency(HRD)status in major cancer types [10]. However, its broader adoption in West China Hospital is constrained primarily by cost; patients from the economically challenged regions of southwest China often find it challenging to afford such specialized medical services. Beyond the economic hurdles, inherent complexities arise when applying NGS to RCC. Prior comprehensive genomic analyses utilizing NGS indicate that RCC typically has a lower frequency of clinically actionable mutations compared to other tumor types; only about 15.3% display at least one targetable alteration [11]. The European Society for Medical Oncology (ESMO) endorses the routine application of NGS for tumor samples in advanced non-squamous non-small-cell lung cancer (NSCLC), prostate cancers, ovarian cancers, and cholangiocarcinoma [12]. Herein, we report our institutional experience in a cohort of 35 RCCs profiled with NGS. We identified the five most frequently mutated actionable genes as PIK3CA, mTOR, ATM, BRCA2, and PALB2, exclusive of mutations in tumor initiators and drivers [13]. These mutational patterns align with prior research findings.
Despite extensive research into the mutation landscape of RCC, the benefits of NGS-guided personalized therapy for Chinese RCC patients still need to be appreciated. In this study, we provided information in a real-world setting. In the context of NGS-guided therapy, an objective remission rate of 41.9% was gained in RCC patients(3 CR, 10 PR), with only 3 PD and four loss of follow-up. In contrast, a phase I clinical trial at MD Anderson documented a 12% ORR in their personalized therapy group [14]. Another prospective trial, focusing on 43 breast cancer patients with targetable genomic alterations who underwent targeted therapy, reported a 9% ORR [15]. Collectively, our findings underscore the potential of NGS testing to address pivotal challenges in precision medicine for RCC.
The current study has several limitations. Firstly, based on Chinese expert consensus on gene testing in renal cell carcinoma [16], we defined potentially beneficial agents for mutated genes. There is a pressing need for future domestic and international high-level guidelines to guide this determination further. Secondly, the implication of NGS in our urologic surgery clinic has been prominently recommended only in the past two years; thus, a discernible advantage in overall survival has yet to be observed. Extensive efforts are warranted to explore precise predictive biomarkers for optimal therapy, and the mutational landscape of Chinese RCC patients on a large scale is an urgent need.
5 Conclusion
The prevalence of implementing NGS after nephrectomy in West China Hospital was reported, with an average of 21% over the last three years. We further incorporated a cohort of 77 Chinese RCC patients. 35 patients received NGS-guided personalized therapy, and 42 underwent empirical therapy. Notably, there was a significant difference in PFS between the two groups. The NGS-guided group showcased an ORR of 41.9% and a DCR of 90.3%. These findings underscore the pivotal role of NGS in advancing precision medicine for RCC.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on request.
References
Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82(5):529–42.
Gansler T, Fedewa S, Amin MB, Lin CC, Jemal A. Trends in reporting histological subtyping of renal cell carcinoma: association with cancer center type. Hum Pathol. 2018;74:99.
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009.
Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67(1):85–97.
Moch H, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2022;82(5):458–68.
Dabestani S, Beisland C, Stewart GD, Bensalah K, Gudmundsson E, Lam TB, et al. Long-term outcomes of follow-up for initially localised clear cell renal cell carcinoma: RECUR database analysis. Eur Urol Focus. 2019;5(5):857–66.
Pontes O, Oliveira-Pinto S, Baltazar F, Costa M. Renal cell carcinoma therapy: current and new drug candidates. Drug Discovery Today. 2022;27(1):304–14.
Navani V, Heng DYC. Treatment selection in first-line metastatic renal cell carcinoma-the contemporary treatment paradigm in the age of combination therapy: a review. JAMA Oncol. 2022;8(2):292–9.
Govindarajan A, Castro DV, Zengin ZB, Salgia SK, Patel J, Pal SK. Front-line therapy for metastatic renal cell carcinoma: a perspective on the current algorithm and future directions. Cancers (Basel). 2022;14(9):2049.
Aleksakhina SN, Imyanitov EN. Cancer therapy guided by mutation tests: current status and perspectives. Int J Mol Sci. 2021;22(20):10931.
Attalla K, DiNatale RG, Rappold PM, Fong CJ, Sanchez-Vega F, Silagy AW, et al. Prevalence and landscape of actionable genomic alterations in renal cell carcinoma. Clin Cancer. 2021;27(20):5595–606.
Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–505.
Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17(4):245–61.
Tsimberidou A-M, Wen S, Hong DS, Wheler JJ, Falchook GS, Fu S, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin Cancer Res. 2014;20(18):4827–36.
André F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014;15(3):267–74.
He D, et al. The Chinese experts consensus of genetic evaluation of kidney cancer (2021 edition). J Modern Urol. 2022;27(3):192–200.
Acknowledgements
We thank patients for their participation in the study.
Funding
This work was supported by the Natural Science Foundation of China (NSFC 82202901, 82203110, 82273047).
Author information
Authors and Affiliations
Contributions
Hao Zeng, Xiang Li, and Zhenhua Liu conceived and designed the study; Yanfeng Tang and Zilin Wang wrote the manuscript; Yanfeng Tang and Yu Shen analyzed the data; Yanfeng Tang, Yu Shen, and Zilin Wang collected samples. All authors have reviewed the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by The Ethical Committee of West China Hospital. All participants provided written informed consent understood the content of the experiment, and agree to publish the article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests, and the manuscript has been approved by all authors for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, Y., Shen, Y., Wang, Z. et al. Next‑generation sequencing‑guided personalized therapy in renal cell carcinoma. Holist Integ Oncol 3, 4 (2024). https://doi.org/10.1007/s44178-024-00072-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00072-1