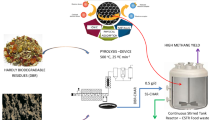
Abstract
This study aims to investigate the significance and biodegradation pathways of PHB-based bioplastic in anaerobic digesters treating food waste, where the reactor performance of changed methane generation, bioplastic biodegradation efficiency, and bioinformatic analysis of functional microbes were emphasized. The results showed that PHB-based plastic film could be partially biodegraded in the food waste digester, and a bioaugmentation use of Alcaligenes Faecalis (AF) and Bacillus Megaterium (BM) was beneficial to largely accelerate the degradation process through a beneficial shift of both the functional bacterial and archaeal species. Microbial community analysis indicated that the major bacterial species belonged to genera Candidatus_Cloacimonas, Rikenellaceae, and Defluviitoga, while the dominant methanogenic archaeal species belonged to genera Methanomassiliicoccus, Methanosarcina, and Methanosaeta. Bioplastic biodegradation analysis suggested that the optimal fractions of AF and BM for PHB-based plastic degradation were 50%AF and 75%BM, respectively, which deserves further optimization and scale-up validation. The finding of this study would contribute to the combined management of PHB-based bioplastic with food waste for clean energy recovery and a greener environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plastics as food packaging materials have been widely used worldwide. The most common types of petroleum-based plastics included high-density polyethylene (HDPE), low-density polyethylene (LDPE), polystyrene (PS), polypropylene (PP), polyethylene terephthalate (PET), and polyvinyl chloride (PVC). Due to the features of high durability and low degradability [1], the extensive use of plastic products has led to serious environmental problems such as white pollution and microplastic issue in both aquatic and terrestrial ecosystems [2, 3]. Without appropriate management from now on, the plastic waste issue would pose huge risks to the public health and sustainable development of human beings. To tackle this issue, many studies have been carried out to look for feasible approaches to reduce plastic pollution, including a reduction in plastic consumption, increase in plastic reuse and recycling, and establishment of safe disposal systems [4]. Even so, more effective solutions need to be developed for the plastic waste issue, especially when the plastic waste was mixed with other heterogeneous wastes.
Regarding the heterogeneous wastes containing plastics, the conventional treatment method in many countries and areas is mainly incineration or landfilling; however, this strategy has been criticized for high carbon emissions and residual contamination (by microplastics and heavy metals) [5]. To fundamentally solve the plastic waste issue, the use of biodegradable plastics instead of petroleum-based plastics has been proposed as a promising solution. Hitherto, in recent years, several kinds of environmental-friendly and degradable plastics such as polyhydroxyalkanoates (PHAs), polylactic acid (PLA) [6], poly(butylene adipate-co-terephthalate) (PBAT) [7], and poly(vinyl alcohol) (PVA) have attracted increasing attention. Among these options, PHAs-based plastic was considered one of the most sustainable option as PHAs can be both bio-derived and biodegradable [8, 9]. Furthermore, it has been found that the energy requirement and CO2 emission involved in the production of bio-derived and biodegradable plastics were significantly lower than those of other classes of plastics [10]. The most extensively studied polymer in the class of PHAs is polyhydroxybutyrate (PHB), which can be completely biodegraded into energy, water, carbon dioxide, and/or methane [11]. The biodegradation of PHB depends on the PHB depolymerase produced by many microorganisms such as Bacillus Megaterium [12, 13] and Alcaligenes Faecalis [14, 15].
Currently, the PHAs-based bioplastic is on the way to be widely commercialized [9]. Nevertheless, seldom studies have been conducted at the practice extent of actual waste management to evaluate the biodegradation performance. For instance, plastics are of especial importance in the food supply chain for food packaging, preservation, transport, hygiene and safety [16]. Hence, bioplastic waste and food waste would concomitantly happen and frequently mixed together, rather than individuals during degradation, namely bioplastic-containing food waste. Currently, anaerobic digestion is a mature technology to convert food waste into methane-rich fuel. However, the reactor performance and effective microbial communities of anaerobic digesters co-treating food waste and bioplastic waste remain unclear. Moreover, the biodegradation kinetics profile of PHB-based plastic in anaerobic digesters remains an essential knowledge gap.
Therefore, the objectives of this study are to investigate the valorization of PHB-based plastic film in the anaerobic digesters of food waste for bioenergy generation, focusing on reactor performance, microbial community analysis, and bioplastic biodegradation. Meanwhile, in order to study the detailed biodegradation process of PHB-based plastic, the degradation rates of PHB films in the fermentation broth of pure Bacillus Megaterium and Alcaligenes Faecalis were closely monitored. The findings of this study would provide technical guidance for the valorization of future PHB-based plastic waste for bioenergy recovery through anaerobic digestion.
2 Materials and methods
2.1 Food waste, seed sludge, chemicals, and commercial strains
The food waste used in this study was collected manually from a campus canteen named Flavours at the National University of Singapore. For the obtained food waste, the non-food components including bones, paper, and plastics were firstly removed. Then, the food waste was homogenized using a household food blender. Seed sludge was provided by a commercial-scale anaerobic digestion institution that co-treated food waste and municipal sewage sludge in Singapore. The characteristics of the seed sludge and food waste were determined according to the respective protocols (shown in Section 2.6), and were summarized in Table 1. The chemicals used for the fabrication of PHB films, including powdered Poly[(R)-3-hydroxybutyric acid] (PHB), glacial acetic acid, and chloroform (co-solvent), were purchased from Sigma-Aldrich Pte Ltd. Two PHB-degradation bacteria, Bacillus Megaterium (BM) and Alcaligenes Faecalis (AF), were purchased from ATCC (American Type Culture Collection) and stored at 4 °C prior to use.
2.2 Preparation of PHB-based bioplastic films
Initially, 0.5 g of PHB powder was weighed and mixed with 13 mL of glacial acetic acid in a 100 mL beaker. The beaker was loosely capped and heated to 40 °C with a magnetic stirrer at 300 rpm. Afterward, the temperature was gradually raised from 40 °C to 150 °C and maintained at 150 °C for 10 min to allow the PHB powder to dissolve completely and to form a translucent and greyish solution. The fabrication of PHB films followed modified procedures from the method by Anbukarasu et al. [17]. Briefly, several microscope glass slides (70 mm × 25 mm) were preheated at 50 °C. Subsequently, 1–1.2 mL of the heated PHB solution (0.04 g/mL) was transferred onto the preheated microscopic glass slide (50 °C). All the microscopic glass slides with PHB solution were heated at 50 °C for appropriately 10 min to dry completely the solution. Then, the microscopic glass slides were cooled down to room temperature (25 °C). Finally, the PHB films were detached from the microscopic glass slides using a pair of tweezers.
2.3 Preparation of microbial fermentation broth for bioplastic degradation experiments
The bacterial strains, Bacillus Megaterium (ATCC14581) and Alcaligenes Faecalis (ATCC8750), were cultivated separately in two 250 mL of flasks (50 mL medium) using the ATCC® Medium 3 (Nutrient agar). The cultivation temperature for Bacillus Megaterium and Alcaligenes Faecalis were 30 °C and 37 °C, respectively. After cultivation for 4 days, the bacterial strains entered the exponential growth period and were subsequently collected for bioplastic degradation experiments.
2.4 Batch anaerobic digestion of food waste with PHB-based plastic
Eight kinds of anaerobic digesters were established using 500 mL of glass bottles, rubber stoppers and gas bags. The anaerobic digesters were inoculated with homogenized seed sludge and other components (e.g., food waste, PHB-based plastic, and BM/AF, see Table 2), and were then capped with rubber stoppers and connected to gas bags. All the digesters were stirred at regular intervals during the entire course of mesophilic (37 °C) anaerobic digestion. At the end of the fermentation, all the gas bags were collected for gas component and volumetric analysis. Each treatment was set in triplicates.
2.5 Biodegradation experiments of PHB films in pure bacterial fermentation broths
Twenty pieces of PHB each weighing 0.05 g were placed in AF and BM solutions of various concentrations, ranging from 100% to 0% (refer to Table 3 for setup). The PHB pieces were weighed regularly and the percentage degradation of PHB over time was plotted.
2.6 Analytical methods
The pH values of liquid samples were tested using a portable pH meter (Agilent 3200 M, USA). Soluble chemical oxygen demand (SCOD) values were measured using a HACH colorimeter (DR900, USA) and the commercial chemical oxygen demand (COD) kits (HC-2125915, Pall Corporation). The TS and VS contents of seed sludge, food waste and bioplastic material were measured via the weighing method from Lin et al. [18]. Carbohydrate content was estimated based on the phenol-sulfuric method [19]. Protein content was determined through the total nitrogen measurement approach coupled with a correction factor of 6.25. Lipid content was determined by the method from Holmes et al. [20]. Elemental compositions were determined by an elemental analyzer (Vario MICRO cube, Germany). The concentration of volatile fatty acids (VFA) was determined with a gas chromatograph (PerkinElmer, Clarus 580 GC, USA) coupled with a flame ionization detector and an auto-sampler.
2.7 Bioinformatic analysis of microbial communities in different digesters
Initially, DNA was extracted by following the protocols reported by Zhang et al. [21]. The DNA concentration and purity were checked by 1% agarose gels electrophoresis. The qualified DNA was diluted to 1 ng/μL with sterile water prior to use. Following that, the polymerase chain reaction (PCR) was performed in order to amplify the V4 region of the 16S rRNA gene using barcoded PCR primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). All PCR reactions were performed using Phusion® High-Fidelity PCR Master Mix (New England Biolabs Inc). Afterward, the sequencing of the DNA samples was carried out through an Illumina HiSeq™ 2000 sequencing system. The raw sequencing data were pretreated to generate effective sequencing reads. Afterward, by following the procedures described by Zhang et al. [21], effective sequences were then used for bioinformatic analysis, including operational taxonomic units (OTUs)-based analysis of the DNA sequences, taxonomic annotation, alpha diversity analysis, and beta diversity analysis. Regarding the taxonomic compositions of microbial communities in different digesters, the dominant bacterial and archaeal species were analyzed at phylum and genus levels. The difference and similarity of microbial community structure among various digesters were visualized by adopting principal coordinates analysis (PCoA).
2.8 Statistical analysis
The Origin software (version 8.5) was used to plot the figures. The variance of the various parameters such as pH, SCOD and methane yield in different digesters were determined statistically through SAS (version 9.4, SAS Institute Inc., USA) coupled with a significance threshold of p < 0.05.
3 Results and discussion
3.1 Performance comparison of different digesters
Regarding the batch anaerobic digestion of food waste with or without PHB-based plastic, a series of parameters including pH, methane yield, methane percentage, CO2 percentage, total nitrogen, SCOD, total volatile fatty acids (TVFA) concentration, and VFAs distribution were determined, results of which are shown in Figs. 1 and 2. From Fig. 1a, the pH values of 7.78 ~ 7.95 showed that all the digesters were operated under normal conditions. From Fig. 1b, the methane yields obtained from the digesters with seed sludge (S), S + food waste (FW), S + FW + AF, S + FW + BM, S + PHB(P1), S + PHB + FW(P2), S + PHB + FW + AF(P3), and S + PHB + FW + BM(P4) were 87.93 ± 32.40, 569.45 ± 88.79, 573.77 ± 89.65, 639.45 ± 42.63, 190.42 ± 30.36, 746.26 ± 79.99, 651.69 ± 67.82, and 586.81 ± 55.92 mL/g VS, respectively. These results had several implications. First, seed sludge (S) itself produced a small portion of methane, which should be considered during the result analysis. Second, among the digesters without PHB, the addition of fermentation broth of AF and BM merely enhanced the methane yields by 0.8 and 12.3%, respectively, indicating that the fermentation broth itself had very limited effects on methane yields. Thirdly, the PHB-based plastic was biodegraded after anaerobic digestion, which led to a methane yield of 102.49 mL/g VS. The results are in accord with previous findings in the literature. Specifically, 25% more methane was obtained from the anaerobic digester treating excess sludge with PHB than that without PHB accumulation [22]. Interestingly, after supplementation of AF or BM fermentation broth, the methane yields decreased by 12.7% and 21.4%, respectively. The decreased methane yields could be ascribed to the PHB biodegradation caused by PHB depolymerase secreted by Bacillus Megaterium and Alcaligenes Faecalis microbes [1, 23]. Moreover, the dynamic methane yields (Fig. 1b) in different digesters could also be caused by the diversified methane percentages (Fig. 1c) and CO2 percentages (Fig. 1d). Specifically, the digesters with S or S + PHB(P1) showed relatively lower methane percentages (i.e., 57.85% and 65.51%) and higher CO2 percentages (i.e., 23.89% and 19.40%) than those of other digesters (i.e., CH4%: 69.89–83.82%; CO2%: 11.45–14.25%). This phenomenon could be attributed to the addition of food waste which led to a more efficient methanogenesis process, resulting in a higher methane percentage and a lower CO2 percentage.
When dealing with nitrogen-containing compounds (e.g., proteins) through AD technology, ammonia derived from biological degradation of nitrogenous matter acts as an important source of nitrogen for microbial growth and is also a potential inhibitor to methanogenesis during AD process [24]. As shown in Fig. 2a, the total nitrogen concentration of these digesters ranged from only 810 to 1160 mg/L, signifying no inhibitory effects of ammonia. It has been reported that a critical point of ammonia concentration for methanogenesis inhibition was 5 g/L [25]. Hence, the digesters of this study were not involved in ammonia inhibition. From Fig. 2b, SCOD values of the digesters with seed sludge (S), S + FW, S + FW + AF, S + FW + BM, S + PHB(P1), S + PHB + FW(P2), S + PHB + FW + AF(P3), and S + PHB + FW + BM(P4) were 1139.5 ± 56.5, 1606 ± 59.30, 1489.67 ± 41.80, 1518.67 ± 60.36, 1585.33 ± 86.61, 1673 ± 61.03, 1345.33 ± 104.08, and 1595.67 ± 32.56 mg/L, respectively. On basis of the total COD of 1.97 ± 0.05 g COD/g VS of food waste, the input COD in the form of food waste was approximately 2.14 g COD in the 300 mL of working volume, equivalent to a COD concentration of 7133.3 mg/L. By calculation, the removed COD of food waste in the digester with S + FW was around 6666.8 mg/L, covering 93.5% of the total COD. The residual COD (approximately 6.5%) comprised the COD in undigested food waste and soluble COD in liquid digestate. Although the addition of PHB, AF, and BF did not significantly alter the SCOD, the concentrations and profiles of VFA were greatly affected. More specifically, the total VFA concentrations of the digesters with seed sludge (S), S + FW, S + FW + AF, S + FW + BM, S + PHB(P1), S + PHB + FW(P2), S + PHB + FW + AF(P3), and S + PHB + FW + BM(P4) were 96.87 ± 13.22, 169.91 ± 57.90, 218.50 ± 49.16, 281.46 ± 93.45, 71.63 ± 11.71, 184.41 ± 61.86, 110.59 ± 15.49, and 229.28 ± 40.99 mg COD/L, respectively. On the one hand, the addition of food waste played a vital role in the total VFA concentration since food waste was the main contributor to the COD (Fig. 2b). On the other, adding AF and BM showed direct effects on VFA distribution (Fig. 2d), which could be ascribed to the selective enrichment of certain microbial communities in AF- or BM-amended digesters (see Section 3.2). It has been reported that bioaugmentation of microbial consortia can significantly change the microbial community structure [26,27,28].
3.2 Comparison of microbial communities in different digesters
3.2.1 Bacterial communities
In order to investigate the valorization of PHB-based plastic film in the anaerobic digesters of food waste for bioenergy generation from the perspective of microbial communities, samples from digesters P1 (S + PHB), P2 (S + PHB + FW), P3 (S + PHB + FW + AF), and P4 (S + PHB + FW + BM) were collected for bioinformatic analysis of bacterial and methanogenic communities in different digesters. The rank-abundance curves (see Appendix A) and the Good’s coverage of 0.998–1.000 jointly proved an adequate relative species abundance and diversity coverage of the sequencing reads. Table 4 shows the alpha diversity index of bacterial and archaeal communities in different digesters. The observed species and the diversity indices (i.e., Shannon, ACE, Chao1, and Simpson indices) among these four digesters displayed different values, signifying that supplementation of food waste, AF, and BM showed varied influences on the microbial community structures. The altered community structures were also validated by the Venn diagram (Fig. 3a) showing both shared and distinctive species among different digesters. Regarding PCoA, the distance matrix of dissimilarities of four data can reflect the difference of beta diversity of various microbial communities. The greater the distance between data points, the greater the difference between them. Results of PCoA analysis (Fig. 3b) indicated that there were major variance in bacterial compositions of digesters P1, P2, P3, and P4.
Venn diagram (a) and PCoA analysis (b) of bacterial communities in the different digesters. P1 for digesters with PHB; P2 for digesters with PHB and food waste; P3 for digesters with PHB, food waste, and AF; P4 for digesters with PHB, food waste, and BM; (c) Taxonomic composition of bacterial communities at the phylum level in different digesters. In the Venn diagram, the values represent the numbers of unique or shared OTUs (operational taxonomic units) among four samples
The taxonomic compositions of bacterial communities in each digester at the phylum are shown in Fig. 3c. The top six major bacterial phyla with a relative abundance of > 3% in digesters P1, P2, P3, and P4 were Bacteroidetes (33.2 ± 7.2%), Cloacimonetes (20.1 ± 13.5%), Firmicutes (24.1 ± 9.7%), Euryarchaeota (5.3 ± 3.3%), Thermotogae (5.0 ± 3.0%), and Proteobacteria (4.8 ± 0.5%), covering 92.5% of the total abundance. These predominant phyla were shared by all the digesters P1, P2, P3, and P4; however, the first predominant phylum was Bacteroidetes (39.4%), Bacteroidetes (40.4%), Cloacimonetes (37.9%), and Firmicutes (32.9%), respectively. Compared to the digesters P1 and P2, the different first dominant bacterial phylum in the digester P3 and P4 caused by bioaugmentation of AF and BM exhibited different effects on AD performance such as methane production. Reportedly, members of Bacteroidetes, Cloacimonetes, Firmicutes, Euryarchaeota, Thermotogae, Proteobacteria, Synergistetes, Cyanobacteria, and Spirochaetes played an important role in the hydrolysis of organic compounds (e.g., proteins and carbohydrates) and production of hydrogen and acetic acid [29,30,31,32]. To further investigate the selective enrichment of bacterial communities in different digesters, the bacterial compositions at the genus level were analyzed.
From Fig. 4, the top predominant (> 3% in relative abundance) bacterial genera in the digesters P1, P2, P3, and P4 were Candidatus_Cloacimonas (20.1 ± 11.7%), Rikenellaceae (18.2 ± 6.8%), and Defluviitoga (4.6 ± 2.6%), respectively. Candidatus_Cloacimonas, a syntrophic bacterial genus found in many anaerobic digesters, is a key player in syntrophic propionate oxidation during AD processes [33]. In this study, the corresponding relative abundance of genus Candidatus_Cloacimonas in the digesters P1, P2, P3, and P4 was 17.8%, 5.0%, 37.8%, and 19.6%, respectively, which indicated that supplementation of AF and BM was beneficial to the enrichment of genus Candidatus_Cloacimonas, led to enhanced syntrophic propionate oxidation in the AD of food waste. Reportedly, the genus Rikenellaceae played a vital role in the anaerobic degradation of organic waste for biogas production [34]. Previously, the genus rikenellaceae has been identified in several other kinds of anaerobic digesters fed with lipid-rich wastewater [35], and distillers grains [36]. After supplementation of AF or BM, the relative abundance of genus Rikenellaceae in the digesters P3 and P4 decreased from 19.4–28.7% to 10.3–14.6%. The variance in the relative abundance of Rikenellaceae among these digesters could be ascribed to the enrichment of other functional species belonging to genus Candidatus_Cloacimonas. The results suggested that the genus Rikenellaceae was an extremely crucial bacteria to a normal AD operation. Belonging to the phylum Thermotogae, genus Defluviitoga was reported as a typical fermentation bacteria responsible for the degradation of carbohydrates and production of volatile fatty acids, hydrogen, and CO2 [31]. Taken together, the relative abundance of the predominant bacteria was affected by the addition of food waste, AF, and BM, compared to the digester P1 with PHB only, leading to diversified digester performances (see Figs. 1 and 2).
3.2.2 Archaeal communities
Venn diagram (Fig. 5a) and PCoA analysis (Fig. 5b) of methanogenic archaeal communities showed that supplementation of food waste, AF, and BM greatly altered the taxonomic compositions of the communities. The relatively long distance between data points P1 and P2 indicated that addition of food waste in the anaerobic digester significantly shifted the microbial community structures. From the data point P2 to data points P3 and P4, the relatively long distances showed that supplementation of AF and BM greatly affected the microbial communities in the digesters. Moreover, the identified archaeal compositions were simpler than the identified bacterial compositions. Taxonomic compositions of archaeal communities at the genus level in different digesters are shown in Fig. 6. Methanogenic archaeal communities included genera Methanomassiliicoccus (42.0 ± 7.5%), Methanosarcina (26.1 ± 11.7%), Methanosaeta (6.9 ± 5.3%), Methanobrevibacter (3.9 ± 1.1%), Methanospirillum (2.2 ± 1.4%), and Methanobacterium (2.1 ± 1.3%), covering 83.3% of total abundance. Regarding the first dominant methanogenic archaeal genus Methanomassiliicoccus, the relative abundance of digesters P1, P2, P3, and P4 was 39.9%, 32.8%, 41.6, and 53.7%, respectively. Methanomassiliicoccus was reported as a hydrogenotrophic methanogenic archaeon capable of synthesizing CH4 by utilizing hydrogen and CO2 or methyl compounds [37, 38]. Degradation of PHB and CO2 generation could be enhanced by the supplementation of AF or BM in the digesters P3 and P4, respectively, compared to the digesters P1 and P2. Hence, the higher relative abundance of genus Methanomassiliicoccus could be beneficial to CO2 utilization via the hydrogenotrophic methanogenesis pathway for methane production. In addition, the genus Methanosarcina was an essential genus for acetate degradation and consumption of H2/CO2 and one-carbon compounds as well as methane production via both hydrogenotrophic and acetoclastic and pathways [39, 40]. Moreover, Methanosarcina was found to be a key methanogenic species related to direct interspecies electron transfer [41,42,43,44]. In this study, although supplementation of AF and BM affected the relative abundance of genus Methanosarcina in the digesters P3 and P4, their relative abundance remains high (e.g., 14.5–44.1%), which played an important role in maintaining the normal methanogenesis processes in various digesters. Additionally, other groups of methanogens such as Methanosaeta synthesize methane using acetic acid, while other minor methanogens such as Methanobrevibacter, Methanospirillum, and Methanobacterium synthesize methane using acetic acid. The mixture of various methanogens with diversified methanogenesis pathways contributed to the utilization of diverse metabolic intermediates during AD of PHB-containing food waste with or without AF or BM, led to a normal methane generation in these digesters (Fig. 1).
Venn diagram (a) and PCoA analysis (b) of archaeal communities in the different digesters. P1 for digesters with PHB; P2 for digesters with PHB and food waste; P3 for digesters with PHB, food waste, and AF; P4 for digesters with PHB, food waste, and BM. In the Venn diagram, the values represent the numbers of unique or shared OTUs (operational taxonomic units) among four samples
3.3 Biodegradation of PHB-based bioplastic in fermentation broth of AF and BM
Figure 7 shows the degradation percentages of PHB-based bioplastic in AF and BM broth. From Fig. 7a, the PHB-based bioplastic with ultra-pure water (i.e., 0%AF and 0%BM groups) did not undergo any reduction in mass, indicating that PHB cannot be degraded in water without the presence of PHB depolymerase enzyme. Regarding PHB degradation in AF broth, the initial rate of PHB degradation with 100% and 75% AF were significantly higher than those of 50% and 25% AF, which could be ascribed to the relatively higher concentration of PHB depolymerase enzyme in 100% and 75% AF broth. To some extent of correlation, the reaction rate was positively proportional to the enzyme concentration [45]. The time to each 50% degradation of PHB-based plastic was approximately 35 d, 55 d, 33 d, and 65 d for 100%AF, 75% AF, 50%AF, and 25%AF broth, respectively. At the 65th day, the degradation percentage of PHB-based plastic was 72.6%, 54.6%, 84.6%, and 52.8% for 100%AF, 75% AF, 50%AF, and 25%AF broth, respectively. These results demonstrated that 50%AF broth can be a promising option for biodegradation of PHB-based plastic. Notably, there was a sharp increase in the degradation rate of PHB-based plastic with 50%AF broth during day 25 to day 35, which could be attributed to the disintegration of PHB-based plastic into several smaller pieces. This particular form of disintegration resulted in a significant increase in the contact area between the bioplastic material and the microorganisms, thus increasing the degradation rate. Reportedly, the depolymerization process of PHB had a close relationship with the availability of nutrients to guarantee optimal growth and metabolic needs [46]. Regarding this, 100%AF broth may not be the optimal condition, which was validated by the experimental results in this study.
As shown in Fig. 7b, for the PHB-based bioplastic biodegradation with fermentation broth of BM, the highest initial degradation rate during day 1 to day 5 was obtained for 100%BM, sequentially followed by 75%BM, 50%BM, and 25%BM, which was accord with the positive correlation between initial degradation rate and the broth concentration. For the 100%BM group, it took around 26 days to reach 50% degradation and about 40 days to reach 75% degradation, demonstrating a higher reaction rate compared to AF (35 days for 50% degradation and 65 days for 75% degradation). Nevertheless, after 1 week of operation, the 75%BM displayed a higher degradation rate than the 100%BM group. This could be due to the fact that 75%BM broth could be a more appropriate concentration to achieve more balanced microbial growth, nutrient consumption, and PHB biodegradation compared to 100%BM broth. More specifically, a healthy microbial growth requires many environmental conditions, including oxygen, nutrition (e.g., carbon source), pH, temperature, and ideal habitat. In this study, the microbial communities grew in a limited experimental growth space (i.e., 50 mL centrifugation tubes). The provided habitat conditions could be sub-optimal for microbes in 100%BM broth due to an excessive number of microorganisms. More studies such as comparison of PHB depolymerase concentration would be carried out in our future work to further illustrate the difference. Furthermore, from day 10 to day 65, the highest degradation rate of PHB-based plastic belonged to BM75% group, sequentially followed by BM100%, BM25%, and BM50%, respectively. Taken together, the appropriate concentration of AF and BM for PHB-based plastic degradation was 50%AF and 75%BM, respectively, which were recommended for scale-up tests. In addition, the same weight of PHB film was used in this study, regardless of film size. Hence, the relationship between PHB size and biodegradation rate needs further investigation.
4 Conclusions
Valorization of PHB-based plastic film in the anaerobic digesters of food waste for methane production was investigated, focusing on reactor performance, microbial community analysis, and bioplastic biodegradation. The results showed that PHB-based plastic could be biodegraded in an anaerobic digester with an average methane yield of 102.49 mL/g VS. The supplementation of Alcaligenes Faecalis (AF) and Bacillus Megaterium (BM) successfully promoted the biodegradation of PHB-based plastic films. The bioinformatic analysis demonstrated that the supplementation of AF and BM greatly altered the bacterial and methanogenic archaeal communities. The major bacterial genera were Candidatus_Cloacimonas (20.1 ± 11.7%), Rikenellaceae (18.2 ± 6.8%), and Defluviitoga (4.6 ± 2.6%), while the dominant methanogenic archaeal genera included Methanomassiliicoccus (42.0 ± 7.5%), Methanosarcina (26.1 ± 11.7%), and Methanosaeta (6.9 ± 5.3%). The results of the biodegradation experiment of PHB-based bioplastic in the fermentation broth of AF and BM indicated that the concentration of 50%AF and 75%BM deserves a recommendation for potential application of PHB-based plastic degradation.
Availability of data and materials
Not applicable.
Abbreviations
- PHB:
-
poly-β-hydroxybutyrate
- AF:
-
Alcaligenes Faecalis
- BM:
-
Bacillus Megaterium
- HDPE:
-
High-density polyethylene
- LDPE:
-
Low-density polyethylene
- PS:
-
Polystyrene
- PP:
-
Polypropylene
- PET:
-
Polyethylene terephthalate
- PVC:
-
Polyvinyl chloride
- PHAs:
-
Polyhydroxyalkanoates
- PLA:
-
Polylactic acid
- PVA:
-
Poly(vinyl alcohol)
- VS:
-
Volatile solids
- TS:
-
Total solids
- SCOD:
-
Soluble chemical oxygen demand
- COD:
-
Chemical oxygen demand
- VFA/VFAs:
-
Volatile fatty acid(s)
- PCR:
-
Polymerase chain reaction
- OTUs:
-
Operational taxonomic units
- PCoA:
-
Principal coordinates analysis
- TVFA:
-
Total volatile fatty acids
- S:
-
Seed sludge
- FW:
-
Food waste
- AD:
-
Anaerobic digestion
References
Wei R, Zimmermann W (2017) Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb Biotechnol 10(6):1308–1322. https://doi.org/10.1111/1751-7915.12710
Chae Y, An Y-J (2018) Current research trends on plastic pollution and ecological impacts on the soil ecosystem: a review. Environ Pollut 240:387–395. https://doi.org/10.1016/j.envpol.2018.05.008
Xanthos D, Walker TR (2017) International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar Pollut Bull 118(1–2):17–26. https://doi.org/10.1016/j.marpolbul.2017.02.048
Lau WW, Shiran Y, Bailey RM et al (2020) Evaluating scenarios toward zero plastic pollution. Science 369(6510):1455–1461. https://doi.org/10.1126/science.aba9475
Shen M, Hu T, Huang W et al (2021) Can incineration completely eliminate plastic wastes? An investigation of microplastics and heavy metals in the bottom ash and fly ash from an incineration plant. Sci Total Environ 779:146528. https://doi.org/10.1016/j.scitotenv.2021.146528
Ren Y, Hu J, Yang M et al (2019) Biodegradation behavior of poly (lactic acid)(PLA), poly (butylene adipate-co-terephthalate)(PBAT), and their blends under digested sludge conditions. J Polym Environ 27(12):2784–2792. https://doi.org/10.1007/s10924-019-01563-3
García-Depraect O, Lebrero R, Rodriguez-Vega S et al (2021) Biodegradation of bioplastics under aerobic and anaerobic aqueous conditions: kinetics, carbon fate and particle size effect. Bioresour Technol:126265. https://doi.org/10.1016/j.biortech.2021.126265
Lambert S, Wagner M (2017) Environmental performance of bio-based and biodegradable plastics: the road ahead. Chem Soc Rev 46(22):6855–6871. https://doi.org/10.1039/c7cs00149e
Tan D, Wang Y, Tong Y et al (2021) Grand challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol 39(9):953–963. https://doi.org/10.1016/j.tibtech.2020.11.010
Harding KG, Dennis JS, von Blottnitz H et al (2007) Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J Biotechnol 130(1):57–66. https://doi.org/10.1016/j.jbiotec.2007.02.012
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56(1):403–432. https://doi.org/10.1146/annurev.micro.56.012302.160838
Chen H-J, Pan S-C, Shaw G-C (2009) Identification and characterization of a novel intracellular poly (3-hydroxybutyrate) depolymerase from Bacillus megaterium. Appl Environ Microbiol 75(16):5290–5299. https://doi.org/10.1128/AEM.00621-09
Gouda MK, Swellam AE, Omar SH (2001) Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res 156(3):201–207. https://doi.org/10.1078/0944-5013-00104
Kasuya K-i, Inoue Y, Yamada K et al (1995) Kinetics of surface hydrolysis of poly[(R)-3-hydroxybutyrate] film by PHB depolymerase from Alcaligenes faecalis T1. Polym Degrad Stab 48(1):167–174. https://doi.org/10.1016/0141-3910(95)00026-I
Nojiri M, Saito T (1997) Structure and function of poly (3-hydroxybutyrate) depolymerase from Alcaligenes faecalis T1. J Bacteriol 179(22):6965–6970. https://doi.org/10.1128/jb.179.22.6965-6970.1997
Hahladakis JN, Iacovidou E (2018) Closing the loop on plastic packaging materials: what is quality and how does it affect their circularity? Sci Total Environ 630:1394–1400. https://doi.org/10.1016/j.scitotenv.2018.02.330
Anbukarasu P, Sauvageau D, Elias A (2015) Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci Rep 5(1):1–14. https://doi.org/10.1038/srep17884
Lin R, Deng C, Ding L et al (2019) Improving gaseous biofuel production from seaweed Saccharina latissima: the effect of hydrothermal pretreatment on energy efficiency. Energy Convers Manag 196:1385–1394. https://doi.org/10.1016/j.enconman.2019.06.044
Masuko T, Minami A, Iwasaki N et al (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339(1):69–72. https://doi.org/10.1016/j.ab.2004.12.001
Holmes CP, Cahill G, Smart KA et al (2013) The composition and ultrastructure of sorghum spent grains. J Inst Brew 119(1–2):41–47. https://doi.org/10.1002/jib.67
Zhang L, Lim EY, Loh KC et al (2020) Biochar enhanced thermophilic anaerobic digestion of food waste: focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers Manag 209:112654. https://doi.org/10.1016/j.enconman.2020.112654
Shamsul HSM, Takashi MHS (2013) Anaerobic digestion of polyhydroxybutyrate accumulated in excess activated sludge. J Water Environ Technol 11(5):429–438. https://doi.org/10.2965/jwet.2013.429
Gamerith C, Herrero Acero E, Pellis A et al (2016) Improving enzymatic polyurethane hydrolysis by tuning enzyme sorption. Polym Degrad Stab 132:69–77. https://doi.org/10.1016/j.polymdegradstab.2016.02.025
Yenigün O, Demirel B (2013) Ammonia inhibition in anaerobic digestion: a review. Process Biochem 48(5–6):901–911. https://doi.org/10.1016/j.procbio.2013.04.012
Yang Z, Wang W, He Y et al (2018) Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renew Energy 125:915–925. https://doi.org/10.1016/j.renene.2018.03.032
Venkiteshwaran K, Milferstedt K, Hamelin J et al (2016) Anaerobic digester bioaugmentation influences quasi steady state performance and microbial community. Water Res 104:128–136. https://doi.org/10.1016/j.watres.2016.08.012
Vinzelj J, Joshi A, Insam H et al (2020) Employing anaerobic fungi in biogas production: challenges & opportunities. Bioresour Technol 300:122687. https://doi.org/10.1016/j.biortech.2019.122687
Yan M, Treu L, Campanaro S et al (2020) Effect of ammonia on anaerobic digestion of municipal solid waste: inhibitory performance, bioaugmentation and microbiome functional reconstruction. Chem Eng J 401:126159. https://doi.org/10.1016/j.cej.2020.126159
Atasoy M, Eyice O, Schnürer A et al (2019) Volatile fatty acids production via mixed culture fermentation: revealing the link between pH, inoculum type and bacterial composition. Bioresour Technol 292:121889. https://doi.org/10.1016/j.biortech.2019.121889
Bassani I, Kougias PG, Treu L et al (2015) Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ Sci Technol 49(20):12585–12593. https://doi.org/10.1021/acs.est.5b03451
Hania WB, Godbane R, Postec A et al (2012) Defluviitoga tunisiensis gen. nov., sp. nov., a thermophilic bacterium isolated from a mesothermic and anaerobic whey digester. Int J Syst Evol Microbiol 62(6):1377–1382. https://doi.org/10.1099/ijs.0.033720-0
Lee J, Han G, Shin SG et al (2016) Seasonal monitoring of bacteria and archaea in a full-scale thermophilic anaerobic digester treating food waste-recycling wastewater: correlations between microbial community characteristics and process variables. Chem Eng J 300:291–299. https://doi.org/10.1016/j.cej.2016.04.097
Dyksma S, Gallert C (2019) Candidatus Syntrophosphaera thermopropionivorans: a novel player in syntrophic propionate oxidation during anaerobic digestion. Environ Microbiol Rep 11(4):558–570. https://doi.org/10.1111/1758-2229.12759
Ozbayram EG, Akyol Ç, Ince B et al (2018) Rumen bacteria at work: bioaugmentation strategies to enhance biogas production from cow manure. J Appl Microbiol 124(2):491–502. https://doi.org/10.1111/jam.13668
Nakasaki K, Nguyen KK, Ballesteros FC et al (2020) Characterizing the microbial community involved in anaerobic digestion of lipid-rich wastewater to produce methane gas. Anaerobe 61:102082. https://doi.org/10.1016/j.anaerobe.2019.102082
Ziganshina EE, Belostotskiy DE, Bulynina SS et al (2021) Effect of magnetite on anaerobic digestion of distillers grains and beet pulp: operation of reactors and microbial community dynamics. J Biosci Bioeng 131(3):290–298. https://doi.org/10.1016/j.jbiosc.2020.10.003
Dridi B, Fardeau ML, Ollivier B et al (2012) Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. Int J Syst Evol Microbiol 62:1902–1907. https://doi.org/10.1099/ijs.0.033712-0
Gonzalez-Martinez A, Garcia-Ruiz MJ, Rodriguez-Sanchez A et al (2016) Archaeal and bacterial community dynamics and bioprocess performance of a bench-scale two-stage anaerobic digester. Appl Microbiol Biotechnol 100(13):6013–6033. https://doi.org/10.1007/s00253-016-7393-z
Ho D, Jensen P, Batstone D (2014) Effects of temperature and hydraulic retention time on acetotrophic pathways and performance in high-rate sludge digestion. Environ Sci Technol 48(11):6468–6476. https://doi.org/10.1021/es500074j
Tao Y, Ersahin ME, Ghasimi DS et al (2020) Biogas productivity of anaerobic digestion process is governed by a core bacterial microbiota. Chem Eng J 380:122425. https://doi.org/10.1016/j.cej.2019.122425
Barros VG, Duda RM, Vantini JS et al (2017) Improved methane production from sugarcane vinasse with filter cake in thermophilic UASB reactors, with predominance of Methanothermobacter and Methanosarcina archaea and Thermotogae bacteria. Bioresour Technol 244:371–381. https://doi.org/10.1016/j.biortech.2017.07.106
De Vrieze J, Hennebel T, Boon N et al (2012) Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour Technol 112:1–9. https://doi.org/10.1016/j.biortech.2012.02.079
Kern T, Fischer MA, Deppenmeier U et al (2016) Methanosarcina flavescens sp. nov., a methanogenic archaeon isolated from a full-scale anaerobic digester. Int J Syst Evol Microbiol 66(3):1533–1538. https://doi.org/10.1099/ijsem.0.000894
Lü F, Luo C, Shao L et al (2016) Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Res 90:34–43. https://doi.org/10.1016/j.watres.2015.12.029
Timmins MR, Lenz RW, Clinton FR (1997) Heterogeneous kinetics of the enzymatic degradation of poly(β-hydroxyalkanoates). Polymer 38(3):551–562. https://doi.org/10.1016/S0032-3861(96)00530-7
Arias S, Bassas-Galia M, Molinari G et al (2013) Tight coupling of polymerization and depolymerization of polyhydroxyalkanoates ensures efficient management of carbon resources in Pseudomonas putida. Microb Biotechnol 6(5):551–563. https://doi.org/10.1111/1751-7915.12040
Acknowledgments
This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) Programme.
Code availability
Not applicable.
Funding
This research was funded by the National Research Foundation, Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) Programme.
Author information
Authors and Affiliations
Contributions
Le Zhang: Conceptualization, Investigation, Data curation, Writing - Original draft preparation, Project management; To-Hung Tsui: Data curation, Writing - Review & Editing; Jiahua Fu: Investigation, Data curation; Yanjun Dai: Writing - Review & Editing, Funding acquisition; Yen Wah Tong: Project management, Writing - Review & Editing, Funding acquisition. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Tsui, TH., Fu, J. et al. Valorization of poly-β-hydroxybutyrate (PHB)-based bioplastic waste in anaerobic digesters of food waste for bioenergy generation: reactor performance, microbial community analysis, and bioplastic biodegradation. Carb Neutrality 1, 8 (2022). https://doi.org/10.1007/s43979-021-00001-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43979-021-00001-5