Abstract
The thermal deep-level transient spectroscopy (DLTS) technique was applied to study the defect states and their activation energies of a polyurethane-based polymer network (PELLE) insulator. It was demonstrated that decomposition analysis of exponential transients allows a more accurate determination of the activation energy of the defective states. Two groups of activation energies for the PELLE polymer were observed, ranging from 0.49 eV to 1.9 eV and − 7.84 eV to − 2.53 eV, which were assigned to changes in the bond properties in the hard and soft segments of the studied polymer, respectively. The applicability of the DLTS method for the characterization of polymers was demonstrated. It was shown that the DLTS technique could contribute to a more comprehensive picture of the properties of polymers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polymers are used in many industries, e.g., photovoltaics and medicine. Therefore, it is interesting to study their properties. The ideal polymer network is electrically neutral over short distances. An imperfection of the polymer network will cause a change in its neutrality. Many structural imperfections or defect groups exist in real polymer structure chains and impurities. Relatively well-illustrated examples of defects are described in [1]. The structural irregularities of different types of polymers often represent vacancies in the structure [2, 3]. These phenomena generate a number of defects that represent local capture or emission centers for charged particles present in the vicinity of such a defect and can be detected and characterized using appropriate techniques. The polymers can be assigned mainly to less-ordered structures. It is appropriate to characterize the density of defect states rather than discrete defect levels.
Radicals, which may be formed in the polymer by breaking bonds by either increased temperature, chemical reaction, or radiation damage, serve as electron capture centers. For example, a material can change its properties due to gamma radiation, high-energy electrons, or other particles [4,5,6]. There are several techniques for studying defects in polymers. Electron spin resonance (ESR) is a widely used technique for characterizing such radicals [7]. Another method is trapping radicals by a chemical reagent [8], nuclear magnetic resonance (NMR) [9], deep-level transient spectroscopy (DLTS) [10], and others. The DLTS is a technique for studying defects, enabling the determination of their activation energies. DLTS has been primarily designed to investigate the properties of semiconductors. DLTS is a non-destructive, relatively fast technique for investigating defects. There are several modifications of DLTS, e.g., capacitance, current, charge, and others. Depending on the physical quantity being processed. The charge DLTS is considered to be the most sensitive [11]. The use of DLTS in the study of polymer–based semiconductors is sporadic and thus far minimal, for example, in [12, 13]. This method has also been used for organic semiconductors [14]. In this study, it is applied to study an organic insulator.
2 Theory
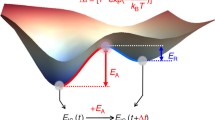
In this article, the so-called temperature DLTS technique was used. The principle of the temperature DLTS modification technique is based on exciting a sample by a DC voltage pulse of a rectangular waveform, in this case, and the temperature variation. At the end of the pulse, the response is monitored. See Fig. 1.
The basic principle of DLTS is the rate window (RW) with t1, t2 and t3 [10]. On the Y-axis is the voltage of the transient phenomena
Generally, the response is of an exponential form, depending on the temperature and material properties. If the DC pulse starts at 0 V, the DLTS signal is obtained by a simplified relationship (1) [10].
τ is the time constant of the measured response exponential signal, which varies with temperature in this case. Tm refers to the temperature when the DLTS reaches a peak or several peaks from the measured temperature range. The parameters, denoted as t1 and t2, define the so-called Rate Window (RW). See Fig. 1 and [10]. If the RW has two parameters, denoted as t1 and t2, then we speak of a so-called 1st -order filter. If another parameter, t3, is added to the RW used, then the RW has three parameters, denoted as t1, t2, and t3, and then we speak of a second-order filter [15, 16]. The 2nd -order filter is more precise in determining energies than the 1st -order filter. In that case, the DLTS signal is described by relationship (2) [16]:
It is advisable to choose the following RW parameters: t0 = 0, t1 ≥ t0, t2 = 2t1, t3 = 4t1, which is the choice we made in Eq. (2), then the calculation DLTS will be simplified when using relationship (2).
The DLTS method processes the transient signal of exponential form. In theory, the transient (of the exponential form) is a superposition of several exponential transients originating from defects (density of states of defects) with different activation energies. Each defect has its activation energy, affecting the exponential response (transient signal). The result is the superposition of several exponentials into one exponential response of the measured signal. For a more precise determination of the activation energies of defect states, the decomposition of the measured DLTS signal (exponential shape) was used. Subsequently, each decomposition component is processed after the decomposition, which is also of exponential form but with different parameters. Several methods exist for decomposing exponential signals, e.g., multiexponential DLTS (MEDLTS) [17].
The measured data were first fitted by a double exponential function using Newton´s numeric minimization of square variance and thus decomposed into two components, short and long. The long component did not show a significant temperature dependence. Finally, the data were smoothed by a moving average to suppress the noise in the transient signal. The two new DLTS plots were obtained from two new exponentials by relation (2).
3 Experiment
In this work, a PELLE (polyurethane-based polymer) (Fig. 2) was chosen as the model polymer due to its interesting structure consisting of crystal blocks (hard domains—within the brackets) and amorphous (soft) domains (outside the brackets).The chemical structure of the polymer is illustrated in Fig. 2. Recently, PELLE material has often been researched and used, e.g., in medicine. The sample was provided by Katharina Ehrmann, Institute of Applied Synthetic Chemistry, TU Wien, Vienna, Austria. More information about this material can be found, e.g., in [18, 19]. The sample was prepared as a flat disc with Au powder on opposite sides. The sample thickness was 0.34 mm, and the diameter was 9.65 mm. It should be noted that the sample was annealed before the DLTS measurement. The sample was annealed two times before measurement. The first annealing was from the ambient temperature to 395 K, and the second annealing was from 100 to 395 K, with a heating step of 10 K and a holding time of approximately 1 h. The annealing of the sample was done in order to prepare the sample by slow cooling of the heated structure, where the primary hard domains formed during the synthesis of the sample were disturbed during the heating, and the following cooling prepared a new semi-crystalline structure with hard and soft domains, which was used for further investigation by the DLTS technique. Then, the sample was measured. Originally, the intention was to analyse the sample with another experimental technique, which motivated the choice of temperature of annealing. In the end, the other experiments were a dead end, so they are not included in the study.
The measuring range of temperatures was from a range approx. 100–405 K. During the measurement run, it was found that it is convenient to go to temperatures of 405 K in order to see the processes even up to this temperature.
The block diagram of the measurement system used is shown in Fig. 3.
The DLTS measurement system block diagram, (1) cryostat with the sample holder, (2) sample, (3) probes of a sample, (4) thermocouple type T, (5) heating resistor, (6) sample heating controller, (7) includes Charge Sensitive Preamplifier (CSP), excitation pulse circuit, circuit of balancing leakage current of the sample, and thermocouple preamplifier, (8) Data Acquisition Card (DAQ), (9) computer
A brief note: The PELLE insulator exhibits a so-called leakage current. The leakage current affects the DLTS spectra and has to be eliminated [20]. Thus, the leakage current of the sample should be eliminated by a suitable design of the CSP circuit. The leakage current sample cannot be eliminated to zero because the bias of the OpAmp in the CSP also contributes to the circuit. If the OpAmp output is eliminated to zero, then the sum of the sample drain current and the OpAmp bias is eliminated. In general, there is no circuit connection that can eliminate sample leakage current to zero. Sample leakage currents are usually on the order of 10− 12 A or less but depend on the sample structure. In this case, the leakage varied in the range from 10− 11to 10− 9 A, depending on the temperature of the sample. The used OpAmp had a bias of 100 fA (10− 15 A), the type was OP129. Therefore, the input bias current of the measurement system, in this case, the charge-sensitive preamplifier (CSP), should be at least 10 times smaller than the sample drain current to obtain DLTS measurement results with sufficient accuracy.
4 Results and discussion
The investigated sample of polyurethane, PELLE, was placed in a cryostat and measured under a vacuum (ca. 10 Pa). The purpose of the vacuum is to prevent frost formation from condensed moisture. All measurements were performed in situ. Individual measurements followed one after the other. One measurement takes about 1 h. Two measurements with an excitation (filling) pulse of (minus) − 9 V and two measurements with an excitation pulse of (minus) − 2 V were performed. The goal was to excite a larger thickness of the sample in order to observe defects in a larger bulk of the sample. The measuring system allows the use of a maximum +/− 9 V excitation voltage. The excitation (filling) pulse period was 100 ms, and the pulse width was 50 ms. The number of measurements and the change in the excitation voltage were chosen to observe how the DLTS signal changes and whether the measurement is reproducible. The sample was cooled in the first step from room temperature to 100 K at a rate of approximately 20 K/min, and then the measurement cycle began to obtain the DLTS signal. Then, the sample was heated during measurement. The temperature range for all heating cycles was approximately 100–405 K. The heating rate was approximately 5 K/min. The DLTS spectra were calculated by relationship (2); seven different RWs were used in each measurement run (100–405 K). Higher temperatures were no longer used due to a change in the state of the sample. The sample used has a melting point of about 420 K. The RW parameters are denoted as t1, t2, and t3. The values of t1 were set as 0.5 ms, 1 ms, 2 ms, 3 ms, 4 ms, 5 ms, and 6 ms (seven RWs). They take into account the period of the excitation pulse and the period. Other RW parameters, t2, and t3, for every seven RWs were determined as described in the text above. Individual measurements were marked as 01_− 9 V, 02_− 9 V, 03_2V, and 04_− 2 V. The first two characters represent the order of measurement. The last characters represent a DC voltage impulse for better orientation. In general, there are many defects and trapped centers for electrons in polymer structures, and their identification is partly possible according to the magnitude of the measured activation energy. The measured values of DLTS spectra represent more significant, large values of defect density, so it is possible to observe them. All DLTS plots were smoothed with a moving average.
In Fig. 4, the DLTS spectra of the first measurement marked 01_− 9 V are illustrated. Seven RWs were measured in one cycle. The growth of the DLTS peak at a larger RW compared to the other peaks is due to the principle of the method itself. The maxima of the spectra at approximately 160 K could not be analyzed because the signal-to-noise ratio of the DLTS signal was low. The same applies to maxima around temperatures of 160 K in all the following figures.
The following Figs. 5, 6, and 7 show the DLTS measurements labeled 02-9V, 03-2V, and 04-2V.
The DLTS plot evolved with every measurement cycle. The peak is shifted on the X-axis (temperature). An Arrhenius plot was used to determine the activation energies of the defect states. The activation energy Wa of electron traps (defects, defect states) in the temperature range of the selected peak can be determined using the Arrhenius plot (Fig. 8). The Arrhenius plot was constructed as Y-axis ln(T 2m .τm) versus X axis 1/kTm, where k is the Boltzmann constant in eV/K. In this case (the 2nd -order filter), the approximation τm ≈ t1 was used [16]. The slope of the linear fit (see Fig. 8) determines the activation energy Wa of electrons trapped in defective areas of the polymer [10]. The smaller maxima can be seen in the DLTS plots of temperature at approximately 160 K, but these maxima could not be analyzed due to the low signal-to-noise ratio. Then, it is more difficult to determine the position of the maximum of the DLTS and, consequently, to construct an Arrhenius plot. The Arrhenius plot at approximately 260 K is shown in Fig. 8. It should be noted that this analysis was performed before decomposition.
Figure 9 shows the construction of the Arrhenius graph from the DLTS spectra at approximately 360 K. The activation energies are shown in Table 1.
As already mentioned, the observed exponential signal is a composition of multiple exponential signals originating from multiple defect states. Simply put, each defect state has its own exponential response. Therefore, it is necessary to decompose these individual states from the measured exponential signal. The measured exponential transient signal was decomposed and subsequently analyzed. In general, decomposition can take place into several components. In this experiment, decomposition into two components was performed, denoted as the 1st and 2nd. Decomposition into more components was not possible because of the relatively low signal-to-noise ratio.
Figure 10 shows the DLTS spectra after the decomposition of the 1st component of the first measurement, labeled 01-9 V. The peak at approximately 310 K cannot be analyzed. It can be assumed that this is caused by the effect of noise on the measured signal. The same applies to maxima around temperatures of 160 K and all the following figures.
The DLTS spectra processed from the second decomposition components are shown in Fig. 11.
Figure 12 shows the DLTS spectra after the decomposition of the 1st component of the first measurement, denoted as 02-9V.
In Fig. 13, we can see the interruptions in the DLTS spectra after decomposition. The interruptions in the graph are a consequence of the already mentioned noise in the measured signal. This can also be seen in other graphs obtained after decomposition. Figures 14, 15, 16 and 17. Illustrated DLTS spectra after the decomposition of the second parts.
In this case, the Arrhenius plots obtained from DLTS after decomposition are illustrated in the following Figures 18, 19, 20 and 21. The energies obtained using the Arrhenius plots significantly differ from those before decomposition. Table 1 shows all the energies obtained from the measurements.
Of course, the shapes of the DLTS spectra are different. After decomposition, it is evident that there were more types of defect density in comparison to the situation when decomposition was not used.
It can be seen that the activation energies changed slightly after each measurement. This slight change in energies can be caused by a change in the structure by the temperature and also by a voltage pulse during the measurement, as mentioned above.
All observed DLTS peaks comes from chain oscillations in the structure. During relaxation, increased chain oscillations occur, which can cause bonds to rupture and thus generate defective states. During measuring (heating from 100 to 405 K), the H bond structure is broken and will not return to its original place, appearing as a defect, for example. The energies at approximately 360 K are from the onset of a phase transformation of the polymer.
After decomposition, the other components from temperatures of approximately 360 K have negative values (see Table 1). In general, the value of Wa involves the interaction of many defect states, both positive and negative. The activation energy is said to be positive because the bond imperfections in the measured structure appear as a positive center in the surrounding microspace (at short distances) and vice versa for negative energy. Therefore, some activation energy values are marked positive and some negative. The column labeled R stands for the correlation coefficient in Table 1.
The decomposition of the exponential DLTS response signal was obtained from two new exponential response signals (transients), in this case, only form two. That’s why there are only two, in this case, because the signal was heavily loaded with noise. Subsequently, each decomposed exponential signal was processed. In general, decomposition may produce one or more decomposed transients. In this experiment, formula (2) was used to obtain the DLTS spectra from each decomposed signal. Subsequently, the activation energy was obtained using the Arrhenius plot. The defect state values after decomposition were investigated. The values obtained from decomposition allowed a more precise analysis of the defect states. We lean towards the following opinions: It is assumed that these are structural defects [2]. The activation energies from a temperature of approximately 360 K evolved with time as the sample was cycled from − 7.84 eV to − 2.53 eV (first to fourth run). This effect is probably caused by a change in the bond properties between O = C in the “hard segment – crystal block” (see Fig. 1 illustrated in [18]). The gradual decrease in the value (− 7.84 eV to − 2.53 eV) of the activation energy is most likely caused by the gradual rearrangement of the structure during individual measurement runs. This change can be caused by temperature (approx. 360 K) under the simultaneous action of the applied voltage and, last but not least, by the material’s properties. A change in bond properties means, for example, any deviation of atoms from the equilibrium position, incomplete bonding, etc. Using this method, the obtained activation values of such bond defects have a minus sign for the following reason. For example, bonding electrons may be missing in the O = C bond in the “hard segment” (see Fig. 2), or the close vicinity of the bond appears positively. If for some reason one more electron would bind there or for some other reason the surroundings would behave negatively, which is also a binding error, the result of solving the Arrhenius plot will be manifested as a negative activation energy. The activation energies with values below 2 eV obtained by the 1st component of decomposition could come from defects located in the “soft segment” (see Fig. 1 from [18]), but they can also come from other segments. This activation energies may come from intramolecular and intermolecular interactions. Most likely, these are disturbances in the bonds, such as dislocations of the atom or even a broken (unsaturated bond between atoms) or a bond between molecules. There is no perfect structure that does not have faults. This statement is valid at all temperatures. Therefore, discontinuous bonds are formed in polymers for some reason, resulting from the formation of defects. The values of bonds between elements in polymers are numerous in the literature and are therefore not even listed here, but one example is in [21]. The DLTS maxima were significant at 160 K, 260 K, and 360 K. The analysis was impossible at temperatures of 160 K, as the DLTS signal was noisy. At 260 K, the signal could be analyzed. It is assumed that the phase transition caused a significant density of defects. For example, the approximation is published in [22,23,24]. Some bonds could rearrange in the structure, and some remained unsaturated. At 360 K, it could be a more pronounced manifestation of the phase transition in the structure. On the one hand, this phenomenon caused defects with values of approximately − 7 eV, as mentioned. Another group of defect states also appeared. The activation energies of these defects were below 2 These defects could be attributed to other bond-related disorders, as mentioned in [20]. The gradual decrease in their activation energies is related to the rearrangement of the PELLE structure.
The activation energies in the “One exponential Wa [eV]” column are determined from the original exponential transient. Energies have ascending values in contrast with the decreasing activation energies obtained after decomposition. We related these energy changes to repeated heating and cooling of the sample and the effects of the DC pulse. The activation energies processed after decomposition are not linearly related to the energies before decomposition. The process is more complicated. Based on this finding, the validity of using decomposition in the DLTS method is confirmed.
5 Conclusions
The aim of the work was to apply the DLTS method to study the defective properties of polymers from the point of view of the properties of the structure and use transient decomposition for a more precise analysis of the activation energies of defect states. It is well known that the DLTS method also allows the study of polarization. It is assumed that the major contribution to the obtained spectra is due to the defects, with a possible minor contribution from polarization.
Significant maxima (peaks) of the DLTS signal were observed at temperatures of 160 K, 260 K, and 360 K. It is possible to examine the general stability of the bonds and to determine the activation energies of their defects by monitoring the capture or emission of electrons in such defects. The decomposition of the transient signal allows an even more accurate determination of the properties of the defect states. In conclusion, the DLTS technique can contribute to a more comprehensive picture of the properties of the studied polymers. In the future, performing decomposition into multiple components may be suitable for defect studies that can be used to improve the electrical and mechanical properties during their fabrication.
Data availability
The data used in this study are available upon request from the corresponding author.
References
Solomon DH, Cacioli P, Moad G. Structural defects in polymers—their identification and significance. Pure Appl Chem. 1985. https://doi.org/10.1351/pac198557070985.
Michler GH, Schmeling HHKBV. The physics and micro-mechanics of nano-voids and nano-particles in polymer combinations. Polymer. 2013. https://doi.org/10.1016/j.polymer.2013.03.035.
Gupta SK, Singh P, Kumar R. Modifications induced by gamma irradiation upon structural, optical and chemical properties of polyamide nylon-6,6 polymer. Radiat Eff Defect in Solid. 2014. https://doi.org/10.1080/10420150.2014.931401.
Ravindrachary IV, Bhajantri RF, Praveena RF, Poojary B, Dutta D, Pujari PK. Optical and microstructural studies on electron irradiated PMMA: a positron annihilation study. Polym Degrad Stab. 2010. https://doi.org/10.1016/j.polymdegradstab.2010.02.031.
Porubská M, Janigová I, Jomová K, Chodák I. The effect of electron beam irradiation on properties of virgin and glass fiber-reinforced polyamide 6. Radiat Phys Chem. 2014. https://doi.org/10.1016/j.radphyschem.2014.04.037.
Porubská M, Szöllős O, Kóňová A, Janigová I, Jašková M, Jomová K, Chodák I. FTIR spectroscopy study of polyamide-6 irradiated by electron and proton beams. Polym Degrad Stab. 2012. https://doi.org/10.1016/j.polymdegradstab.2012.01.017G.E.
Pake EH. Paramagnetic resonance: an introductory monograph. New York: W.A. Benjamin; 1962.
March J. Advanced organic chemistry: reactions, mechanisms, and structure. 3rd ed. New York: Wiley; 1985.
Khramtsov V, Berliner LJ, Clanton IT. NMR spin trapping: detection of free radical reactions using a phosphorus-containing nitrone spin trap. Magn Reson Med. 1999;1:1.
Lang DV. Deep-level transient spectroscopy: a new method to characterize traps in semiconductors. In J App Phys. 1974. https://doi.org/10.1063/1.1663719.
Kirov KI, Radev KB. A simple charge-based DLTS technique. Phys Stat Solidi. 1981. https://doi.org/10.1002/pssa.2210630241.
Hsieh H-C, Hsiow C-Y, Lin K-F, Shih Y-C, Wang L. Cédric Renaud, and Thien-Phap Nguyen. Analysis of defects and traps in N–I–P layered-structure of Perovskite Solar cells by charge-based deep level transient spectroscopy (Q-DLTS). J Phys Chem C. 2018. https://doi.org/10.1021/acs.jpcc.8b01949.
Campbell AJ, Bradley DDC, Werner E, Brütting W. Deep level transient spectroscopy (DLTS) of a poly(p-phenylene vinylene) Schottky diode. Synth Met. 2000. https://doi.org/10.1016/S0379-6779(99)00446-4.
Stuchlíková L, Weis M, Juhász P, Kósa A, Harmatha L, Jakabovic J. Defect analysis of Pentacene Diode. Acta Phys. 2014. https://doi.org/10.12693/APhysPolA.125.1038.
Crowell CR, Alipanahi S. Transient distortion and nth order filtering in deep level transient spectroscopy (DnLTS). In Solid-State Elektron. 1981. https://doi.org/10.1016/0038-1101(81)90209-4.
Thurzo I, Teramura S, Durný R, Nádaždy V, Kumeda M, Shimizu T. Small-signal deep level transient spectroscopy on hydrogenated amorphous silicon based metal/insulator/semiconductor structures. J App Phys. 1998. https://doi.org/10.1063/1.368978.
Morimoto J, Kida T, Miki Y, Miyakawa T. Multi-exponential analysis of DLTS. Appl Phys A. 1986. https://doi.org/10.1007/BF00620735.
Mrad O, Saunier J, Aymes Chodur C, Rosilio V, Agnely F, Aubert P, Vigneron J, Etcheberry A, Yagoubi N. A comparison of plasma and electron beam-sterilization of PU catheters. Radiat Phys Chem. 2010. https://doi.org/10.1016/j.radphyschem.2009.08.038.
Seidler K, Ehrmann K, Steinbauer P, Rohatschek A, Andriotis OG, Dworak C, Koch T, Bergmeister H, Grasl C, Schima H, Thurner PJ, Liska R, Baudis S. A structural reconsideration: linear aliphatic or alicyclic hard segments for biodegradable thermoplastic polyurethanes? Polym Chem A. 2018. https://doi.org/10.1002/pola.29190.
Chen MC, Lang DW, Dautremont-Smith W, C,Sergent AM, Harbison JP. Effects of leakage current on deep level transient spectroscopy. App Phys Lett. 1984. https://doi.org/10.1063/1.94887.
Golbert M. Brydson's plastics materials. 8th edn. Elsevier; 2016
Chun-Qing H, Yi – Qun D, Bo W, Sao-Jie W, Gui-You W, ChunPu H. Temperature depence of free volume in cross-linked polyurethane studied by positrons. Chin Phys Lett. 2001. https://doi.org/10.1088/0256-307X/39/1/017402.
Siani A, Novak A, Rodier L, Eeckhaut G, Leeslang J-W, Higgins JS. Origin of multiple melting endotherms in a high hard block content polyurethane: effect of annealing temperature. Macromolecules. 2007. https://doi.org/10.1021/ma070332p.
Ehrmann K, Potzmann P, Dworak C, Bergmeister H, Eilenber M, Grasl C, Koch T, Schima H, Liska R, Baudis S. Hard block degrable polycarbonate urethanes: promising biomaterials for electrospun vascular prostheses. Biomacromolecules. 2020. https://doi.org/10.1021/acs.biomac.9b01255.
Acknowledgements
The authors thank Katharina Ehrmann from the Institute of Applied Synthetic Chemistry, TU Wien, Austria, for the preparation of the PELLE sample and to Slovak scientific agency VEGA, Grant No. 2/0024/17, and VEGA 2/0162/22, and APVV-21-0335 for financial support.
Author information
Authors and Affiliations
Contributions
The coordinator and author of the whole idea is Jaroslav Rusnak. Mr. Michal Pecz participated in the method of decomposition. Dr. Jan Skoviera participated in the interpretation of the measured results. Mr. Ivan Klbik participated in the interpretation of the measured results. All authors expressed their support for the publication. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the content of the article is not in any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rusnák, J., Pecz, M., Škoviera, J. et al. Thermal DLTS study of the defect states of PELLE polymer. Discov Mater 3, 22 (2023). https://doi.org/10.1007/s43939-023-00059-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-023-00059-1