Abstract
Objective
Anterior shoulder dislocations are commonly treated in the emergency department (ED). Analgesia for reduction is provided by intra-articular lidocaine (IAL) injection or intravenous sedation (IV sedation). The objective of this systematic review and meta-analysis was to compare IAL versus IV sedation for closed reduction of acute anterior shoulder dislocation in the ED.
Methods
Electronic searches of MEDLINE and EMBASE (1946–September 2021) were completed and reference lists were hand-searched. Randomized controlled trials (RCTs) comparing IAL and IV sedation for reduction of acute anterior shoulder dislocations among patients ≥ 15 years old in the ED were included. Outcomes of interest included a successful reduction, adverse events, ED length of stay, pain scores, procedure time, ease of reduction, patient satisfaction, and cost. Two reviewers independently screened abstracts, assessed study quality and extracted data. Data were pooled using random-effects models and reported as mean differences and risk ratios (RR) with 95% confidence intervals (CIs).
Results
12 RCTs were included with a total of 630 patients (IAL = 327; IV sedation = 303). There was no difference in reduction success between IAL and IV sedation (RR 0.93; 95% CI 0.86–1.01, I2 = 69%), significantly lower adverse events with IAL (RR 0.16; 95% CI 0.07–0.33, I2 = 0%), shorter ED length of stay with IAL (mean difference − 1.48; 95% CI − 2.48 to − 0.47, I2 = 93%), no difference in pain scores post-analgesia and no difference in ease of reduction.
Conclusions
Intra-articular lidocaine may have similar effectiveness as IV sedation in the successful reduction of anterior shoulder dislocations in the ED with fewer adverse events, shorter ED length of stay, and no difference in pain scores or ease of reduction. Intra-articular lidocaine may be an effective alternative to IV sedation for reducing anterior shoulder dislocations, particularly when IV sedation is contraindicated or not feasible.
Résumé
Objectif
Les luxations antérieures de l'épaule sont couramment traitées au service des urgences (SU). L'analgésie pour la réduction est fournie par une injection intra-articulaire de lidocaïne (IAL) ou par une sédation intraveineuse (sédation IV). L'objectif de cette revue systématique et méta-analyse était de comparer la sédation IAL par rapport à la sédation IV pour la réduction fermée de la luxation antérieure aiguë de l'épaule aux urgences.
Méthodes
Des recherches électroniques ont été effectuées sur MEDLINE et EMBASE (1946-septembre 2021) et les listes de références ont été consultées manuellement. Les essais contrôlés randomisés (ECR) comparant la sédation IAL et IV pour la réduction des luxations antérieures aiguës de l'épaule chez les patients ≥ 15 ans aux urgences ont été inclus. Les résultats d'intérêt comprenaient une réduction réussie, les effets indésirables, la durée de séjour aux urgences, les scores de douleur, la durée de la procédure, la facilité de réduction, la satisfaction du patient et le coût. Deux examinateurs ont indépendamment passé en revue les résumés, évalué la qualité des études et extrait les données. Les données ont été regroupées à l'aide de modèles à effets aléatoires et présentées sous forme de différences moyennes et de rapports de risque (RR) avec des intervalles de confiance (IC) à 95 %.
Résultats
12 ECR ont été inclus avec un total de 630 patients (IAL = 327 ; sédation IV = 303). Il n’y avait pas de différence dans le succès de réduction entre la sédation IAL et la sédation IV (RR = 0,93; IC à 95 % : 0,86 à 1,01, I2 = 69 %), événements indésirables significativement plus faibles avec IAL (RR = 0,16; IC à 95 % : 0,07 à 0,33, I2 = 0 %), durée de séjour plus courte avec IAL (différence moyenne = -1,48; IC à 95 % : -2,48 à -0,47, I2 = 93 %), aucune différence dans les scores de douleur après l’analgésie et aucune différence dans la facilité de réduction.
Conclusions
La lidocaïne intra-articulaire peut avoir une efficacité similaire à celle de la sédation IV dans la réduction réussie des luxations antérieures de l'épaule aux urgences avec moins d'effets indésirables, une durée de séjour aux urgences plus courte et aucune différence dans les scores de douleur ou la facilité de réduction. La lidocaïne intra-articulaire peut être une alternative efficace à la sédation IV pour réduire les luxations antérieures de l'épaule, en particulier lorsque la sédation IV est contre-indiquée ou impossible.
Similar content being viewed by others
What is known about the topic? |
Several randomized controlled trials have compared intra-articular lidocaine (IAL) and intravenous sedation (IV sedation) for anterior shoulder dislocation reductions. |
What did this study ask? |
What is the effectiveness of IAL versus IV sedation on closed reduction of acute anterior shoulder dislocation in the ED? |
What did this study find? |
IAL had similar reduction success and pain scores as IV sedation, with fewer adverse events and shorter length of stay. |
Why does this study matter to clinicians? |
IAL may be an effective alternative for shoulder reduction when IV sedation is contraindicated or is not feasible (solo-coverage/low-resourced EDs). |
Introduction
Anterior shoulder dislocations represent 95% of all shoulder dislocations, and is the most common type of joint dislocation seen in the emergency department (ED) [1, 2]. Treatment of anterior shoulder dislocations involves relocating the humeral head within the glenoid fossa, using one of several previously described reduction techniques [3,4,5,6]. Imperative to the success of reduction is muscle relaxation and patient cooperation [2]. As such, analgesia is often required.
Intra-articular lidocaine and intravenous sedation (IV sedation) are two types of analgesia for anterior shoulder dislocation reduction [2]. There have been several randomized controlled trials (RCTs) comparing these two methods in the ED setting [7,8,9,10,11,12,13,14]. Despite the popularity of IV sedation, intra-articular lidocaine has been shown to have similar rates of successful reduction with fewer systemic complications and decreased costs [15]. In a 2014 systematic review and meta-analysis by Jiang et al., intra-articular lidocaine was found to be similar in reduction efficacy compared to IV sedation with an improved safety profile [16], despite the inclusion of a trial reporting a lower rate of successful reduction and decreased patient satisfaction with intra-articular lidocaine [17]. Additionally, the American College of Emergency Physicians have a toolkit entitled, ‘Managing Acute Pain’ which suggests intra-articular lidocaine may be equivalent to IV sedation for successful reduction of acute anterior shoulder dislocations [18].
Recently, three new trials were published comparing intra-articular lidocaine and IV sedation [19,20,21]. The primary objective of this systematic review and meta-analysis was to compare the efficacy of intra-articular lidocaine and IV sedation for successful closed reduction of acute anterior shoulder dislocation in the ED. Secondary outcomes of interest include adverse events, ED length of stay, change in pain scores, procedure time, ease of reduction, patient satisfaction, and cost.
Methods
Data sources and search strategy
In consultation with the review authors, a research librarian conducted the systematic literature searches in MEDLINE (1946–September 2021) using both Ovid and PubMed search interfaces, EMBASE (1947–September 2021), the Cochrane Central Register of Controlled Trials (September 2021), as well as electronic bibliographic databases. A comprehensive search strategy (see supplementary material for search strategy) included a combination of medical subject headings (MeSH) and free-text terms using various spelling and endings of key words. The protocol for this systematic review and meta-analysis was not registered online.
Eligibility criteria and study selection
RCTs published comparing the use of intra-articular lidocaine versus IV sedation (any agent) for the closed reduction of acute anterior shoulder dislocations among patients ≥ 15 years of age in the ED were eligible for inclusion. Studies involving only pediatric populations, posterior shoulder dislocations, fracture-dislocations, or settings other than the ED were excluded. Two reviewers independently screened the search output to identify potentially eligible trials. Corresponding full texts were then retrieved and assessed for inclusion. In the event of discrepancies, a third researcher adjudicated the decision. Reference lists of relevant articles and the regulatory website ‘clinicaltrials.gov’ were searched to identify any unpublished or missed RCTs.
Outcome measures
A standardized data collection form was used to extract data on patient demographics (country of study, sample size, patient age), intervention (intra-articular lidocaine versus IV sedation agents, concentrations, and doses if available) and outcomes. Data for each study was extracted by one researcher and verified by a second researcher. In the event of discrepancies, a third researcher adjudicated the decision. Outcomes of interest included a successful reduction, adverse events, ED length of stay, pain scores, procedure time, ease of reduction, patient satisfaction, and cost. We defined a successful reduction as post-reduction radiological confirmation of shoulder relocation. Monitored adverse events varied across trials, and for meta-analysis we included the presence or absence of adverse events as dictated by the individual RCTs. Pain differences were calculated from raw pain scores to provide pain reduction scores for meta-analysis. Pain scores were included if patient-reported pain was described on a 10-point scale. Procedure time was defined as the time for reduction, not the administration of anesthesia or length of stay post-reduction. Cost data were summarized narratively. Where raw data were required for meta-analyses and not derivable or reported, the authors were contacted in efforts to obtain this data.
Risk of bias assessment
Risk of bias for each individual trial was independently assessed by two reviewers using the Cochrane Collaboration’s tool for assessing risk of bias for systematic reviews of interventions [22]. We assessed the risk of bias for each study using the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each domain was assessed as having a low, unclear, or high risk of bias. Discrepancies were resolved by discussion and consensus amongst the authors.
Data synthesis and analysis
Direct comparisons of ED length of stay (in hours), pain score after anesthesia and before reduction, reduction in pain score, and procedure time (in minutes) were performed using inverse variance random-effects models to account for both within study and between study heterogeneity, and reported as mean differences with 95% confidence intervals (CIs) using Review Manager 5.3.4 (Nordic Cochrane Centre, Copenhagen, Denmark). A mean difference < 0 favored intra-articular lidocaine and statistical significance was achieved if the 95% CI of the pooled estimate excluded zero. Direct comparisons of dichotomous outcomes (successful reduction, patient satisfaction, adverse events, and provider ease of reduction) were performed using Mantel–Haenszel random-effects models and reported as risk ratios (RR) with 95% CIs. RRs > 1 favored intra-articular lidocaine, and statistical significance was achieved if the 95% CI of the pooled RR excluded unity. Statistical heterogeneity between studies was assessed using the I2 statistic, with I2 values ≥ 50% indicating substantial heterogeneity. We also conducted a subgroup analysis separating studies comparing meperidine/pethidine to intra-articular lidocaine as this drug is no longer used.
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach which provides a structured and transparent framework to assess quality (high, moderate, low and very low) of the evidence [23]. We used conventional GRADE guidance and considered risk of bias, inconsistency, imprecision, indirectness, and publication bias for the body of evidence informing each outcome.
Results
Study selection and characteristics
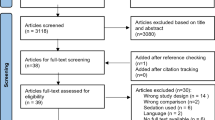
The search strategy yielded 554 citations. After eliminating duplicates and screening studies that did not meet eligibility criteria, 23 studies remained for full-text review (Fig. 1). A total of 12 RCTs were included with a total of 630 patients (327 patients in the intra-articular lidocaine group and 303 patients in the IV sedation group) [8,9,10,11,12,13,14, 17, 19,20,21, 24]. A summary of trial characteristics is shown in Table 1. All studies were published between 1994 and 2020 involving ED populations from 6 countries and ages ranged from 16 to 89 years. One study was reported as an abstract, but additional data was obtained from the authors [20]. The intra-articular lidocaine group all received 1% lidocaine, with 2 trials administering 4 mg/kg and the remaining 10 trials administering 20 mL of lidocaine (regardless of body weight). The IV sedation groups across all trials received different medication combinations (anxiolytics, analgesics, or sedatives) and doses. Some trials had pre-determined medications and doses for IV sedation, while others were weight-based or up to the discretion of the treating physician. Risk of bias varied across trials (Table 2). All trials were found to have a low risk of bias regarding outcome data and reporting, and high risk of bias with regards to blinding of patients and providers.
Outcomes
Successful reduction
All 12 studies, with a total of 630 patients (327 patients in the intra-articular lidocaine group and 303 patients in the IV sedation group) reported on reduction success (Fig. 2) [8,9,10,11,12,13,14, 17, 19,20,21, 24]. The pooled estimate showed no difference in reduction success between intra-articular lidocaine and IV sedation (83.8% vs. 91.4%; RR 0.93; 95% CI 0.86–1.01, I2 = 69%, low certainty). The low certainty in evidence on GRADE assessment was a result of inconsistency across trials and non-blinding (Table 3). The subgroup analysis (supplementary Fig. 1) removing studies comparing meperidine/pethidine to intra-articular lidocaine did not a significant change in reduction success (intra-articular lidocaine: 83.8% vs. IV sedation without meperidine/pethidine 95.7%; RR 0.91; 95% CI 0.78–1.05, I2 = 80%).
Adverse events
Eleven studies, with a total of 586 patients reported adverse events (Supplementary Fig. 2) [8,9,10,11,12,13,14, 17, 19, 21, 24]. The pooled estimate showed a difference in adverse events, with fewer occurring in the intra-articular lidocaine group compared to the IV sedation group (1.3% vs. 20.8%; RR 0.16; 95% CI 0.07–0.33, I2 = 0, moderate certainty). Two studies reported an adverse event in the intra-articular lidocaine groups, which included agitation and drowsiness. Adverse events reported in the IV sedation groups ranged from: respiratory depression (including apnea/hypoxia), hypotension, nausea/vomiting, headache, allergic reaction, and thrombophlebitis.
ED length of stay
Seven studies, with a total of 299 patients reported on ED length of stay (Supplementary Fig. 3) [8, 9, 11, 12, 17, 20, 24]. The pooled estimate showed a significant difference in mean ED length of stay with a shorter duration in the intra-articular lidocaine group compared to the IV sedation group (mean difference = − 1.48 h; 95% CI − 2.48 to − 0.47, I2 = 93%, moderate certainty).
Pain scores
Eight studies, with a total of 444 reported on pain scores after anesthesia and before reduction (Supplementary Fig. 4) [9,10,11,12,13,14, 19, 24]. The pooled estimate showed no difference in pain score after anesthesia and before reduction in the intra-articular lidocaine group compared to the IV sedation group (mean difference = − 0.04; 95% CI − 1.10 to 1.02, I2 = 96%, very low certainty). Four studies, with a total of 263 patients reported on the change in pain scores pre-anesthesia and post-reduction (Supplementary Fig. 5) [8, 9, 19, 24]. The pooled estimate showed no difference in the change in pain scores pre-anesthesia and post-reduction in the intra-articular lidocaine group compared to the IV sedation group (mean difference = − 0.29; 95% CI − 1.08 to 0.5, I2 = 0%, low certainty).
Procedural time
Five studies, with a total of 243 patients reported on the length of procedural time for reduction to occur (Supplementary Fig. 6) [9,10,11, 13, 21]. The pooled estimate showed a significant difference with increased procedural time in the intra-articular lidocaine group compared to IV sedation (mean difference = 8 min; 95% CI 4.42–11.57, I2 = 97%, moderate certainty).
Ease of reduction
Five studies, with a total of 233 patients reported on provider ease of reduction (Supplementary Fig. 7) [8, 12, 17, 21, 24]. The pooled estimate showed no statistically significant difference in ease of reduction in the intra-articular lidocaine group compared to IV sedation (54.5% vs. 71.8%; RR 0.78; 95% CI 0.59–1.04, I2 = 51%, very low certainty). The very low certainty in evidence on GRADE assessment for pain scores and ease of reduction was a result of inconsistency, imprecision, and non-blinding (Table 3).
Patient satisfaction
Six studies, with a total of 380 patients reported on patient satisfaction (Supplementary Fig. 8) [8, 10, 13, 17, 19, 24]. The pooled estimate showed a significant difference with decreased patient satisfaction in the intra-articular lidocaine group compared to IV sedation (70.5% vs. 90.4%; RR 0.80; 95% CI 0.67–0.95, I2 = 71%, moderate certainty).
Cost
The cost of intra-articular lidocaine was reported as less than IV sedation in four trials: $0.52 United States Dollars (USD) versus $97.64 USD [11], $117–$133 USD versus $159.55–$240.55 USD [12], 150 Nepalese Rupees (NPR) versus 400 NPR [21], and $10 USD versus $31 USD [24].
Discussion
In this systematic review and meta-analysis including 12 studies, with a total of 630 patients (327 patients in the intra-articular lidocaine and 303 patients in the IV sedation group), we found intra-articular lidocaine may have similar effectiveness as IV sedation in the successful reduction of anterior shoulder dislocations in the ED with fewer adverse events, shorter ED length of stay, and no difference in pain scores or ease of reduction. However, patient satisfaction was lower with intra-articular lidocaine.
Our findings were in agreement with previous systematic reviews, demonstrating the benefits of intra-articular lidocaine [16, 26,27,28,29]. In a 2011 systematic review and meta-analysis by Wakai et al., comparing the clinical efficacy and safety of intra-articular lignocaine and intravenous analgesia (with or without sedation) for reduction of acute anterior shoulder dislocation, there was no significant difference between intra-articular lidocaine and IV sedation with regard to the immediate success rate of reduction, pain during reduction, post-reduction pain relief and reduction failure [27]. Similar to our findings, the authors suggested intra-articular lidocaine may be less expensive and may be associated with fewer adverse effects and a shorter recovery time, compared to IV sedation. More recently, Jiang et al., included data from nine RCTs with a total of 438 patients and found intra-articular lidocaine may be safer than IV sedation as there were fewer complications with intra-articular lidocaine. Both techniques were similarly effective for manual closed reduction of acute anterior shoulder dislocation [28].
Strengths and limitations
Strengths of this review include explicit eligibility criteria, a comprehensive search, and independent duplicate assessment of eligibility. This is the most up to date systematic review and meta-analysis on this topic, and we used the GRADE approach which provides a structured and transparent framework to assess quality of the evidence [23].
This systematic review and meta-analysis has several limitations. As with all meta‐analyses, the results from this study are limited by the quality of trials included. One included trial was an abstract [20] that was unlikely to be peer reviewed to the same level of scrutiny as the full‐text journal articles. However, this was highlighted through our assessments showing high or unclear risks of bias in study methodology, varying or unclear definitions of outcomes, and how they were measured. We did not search grey literature for potential studies. However, we would suspect that the majority of RCTs would also be published and found in the databases searched. Many of the outcomes we assessed in the meta-analysis had a high I2 values, including the success of reduction, indicating significant heterogeneity between studies, which may in turn limit the application of our results. Furthermore, four outcomes (successful reduction, both pain score outcomes, and ease of reduction) had a low to very low certainty of evidence. Another limitation was the inconsistency in the type of IV sedation agents used both within and between trials. Six trials used meperidine/pethidine (Demerol) in combination with a benzodiazepine or propofol [8,9,10, 13, 21, 24]. The remaining six trials used propofol, etomidate, ketamine, morphine, fentanyl, midazolam and diazepam [11, 12, 14, 17, 19, 20]. Given the different mechanisms of action, this could influence potential adverse events and subsequent risk of occurrence. Finally, the success of intra-articular lidocaine may be influenced by provider skill, and most trials did not comment on physician comfort with shoulder joint injections [25].
Clinical implications
Intra-articular lidocaine and IV sedation have different advantages based on the ED setting in which a physician is working and are both useful skills for emergency medicine providers. Our findings suggest that IV sedation is associated with improved patient satisfaction and reduced procedural time. IV sedation may be the preferred option in a well-resourced setting when treating a patient who has previously had a difficult reduction, has significant apprehension to being alert during the procedure, has local anesthetic allergy or toxicity risk, or when the provider has discomfort with joint injection technique. Although intra-articular lidocaine may be associated with less patient satisfaction, intra-articular lidocaine may be beneficial to consider in several settings, including in low-resourced settings (e.g., single coverage EDs or remote communities) where access to IV sedation drugs or equipment may be limited or not feasible [29]. Additionally, in EDs where healthcare dollars are limited (e.g., low-income communities/countries, patients who must pay for treatment out of pocket), intra-articular lidocaine may offer similar clinical outcomes to IV sedation at a lower cost [30, 31]. In high volume EDs with bed shortages, intra-articular lidocaine may be considered advantageous for departmental flow given the decreased ED length of stay compared to IV sedation [32, 33]. Additionally, we found that there were fewer adverse events with intra-articular lidocaine compared to IV sedation. Commonly used IV sedation agents such as propofol and opioids, have several well-known side effects such as hypotension, apnea, nausea and vomiting [35, 36]. The most commonly reported adverse event from IV sedation in the included RCTs was respiratory depression [8,9,10, 13, 19, 24]. Given the potential adverse events associated with IV sedation, intra-articular lidocaine may be favorable in patients who are complex, clinically unstable, or thought to have predicted difficult airway. Also, due to the risk of apnea and respiratory depression, many EDs require a respiratory therapist or additional physician for procedural sedation. This this can be strenuous on busy or solo-coverage EDs. Bed shortages, resource scarcity, and precautions around potential aerosol generating medical procedures have been accentuated during the COVID-19 pandemic, further providing settings where intra-articular lidocaine may be considered alongside IV sedation [33, 34, 37].
Conclusions
In this systematic review and meta-analysis of 12 RCTs with 630 patients, we found that intra-articular lidocaine may have similar effectiveness as IV sedation in the successful reduction of anterior shoulder dislocations in the ED with fewer adverse events, shorter ED length of stay, and no difference in pain scores or ease of reduction. Intra-articular lidocaine may be an effective alternative to IV sedation for reducing anterior shoulder dislocations, particularly when IV sedation is contraindicated or not feasible.
References
Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Jt Surg Am. 2010;92:542.
Ziang N, Hu YJ, Zhang KR, et al. Intra-articular lidocaine versus intravenous analgesia and sedation for manual closed reduction of acute anterior shoulder dislocation: an updated meta-analysis. J Clin Anesth. 2014;26:350–9.
Baykal B, Sener S, Turkan H. Scapular manipulation technique for reduction of traumatic anterior shoulder dislocations: experiences of an academic emergency department. Emerg Med J. 2005;22:336.
Eachempati KK, Dua A, Malhotra R, et al. The external rotation method for reduction of acute anterior dislocations and fracture-dislocations of the shoulder. J Bone Jt Surg Am. 2004;86-A:2431.
O’Connor DR, Schwarze D, Fragomen AT, et al. Painless reduction of acute anterior shoulder dislocations without anesthesia. Orthopedics. 2006;29:528.
Cunningham NJ. Techniques for reduction of anteroinferior shoulder dislocation. Emerg Med Australas. 2005;17:463–71.
Lippit S. Treatment of shoulder dislocations with lidocaine. Anesthesiol Top. 1991;10:6.
Orlinsky M, Shon S, Chiang C, et al. Comparative study of intra-articular lidocaine and intravenous meperidine/diazepam for shoulder dislocations. J Emerg Med. 2002;22:241–5.
Moharari RS, Khademhosseini P, Espandar R, et al. Intra-articular lidocaine versus intravenous meperidine/diazepam in anterior shoulder dislocation: a randomized clinical tr. Emerg Med J. 2008;25:262–4.
Suder PA, Mikkelsen JB, Hougaard K, et al. Reduction of traumatic primary anterior shoulder dislocation under local analgesia [article in Danish]. Ugeskr Laeger. 1995;157:3625–9.
Miller SL, Cleeman E, Auerbach J, et al. Comparison of intraarticular lidocaine and intravenous sedation for reduction of shoulder dislocations: a randomized, prospective study. J Bone Jt Surg Am. 2002;84-A:2135–9.
Matthews DE, Roberts T. Intraarticular lidocaine versus intravenous analgesic for reduction of acute anterior shoulder dislocations. A prospective randomized study. Am J Sports Med. 1995;23:54–8.
Suder PA, Mikkelsen JB, Hougaard K, et al. Reduction of traumatic secondary shoulder dislocations with lidocaine. Arch Orthop Trauma Surg. 1995;114:233–6.
Kosnik J, Shamsa F, Raphael E, et al. Anesthetic methods for reduction of acute shoulder dislocations: a prospective randomized study comparing intraarticular lidocaine with intravenous analgesia and sedation. Am J Emerg Med. 1999;17:566–70.
Ng VK, Hames H, Millard WM. Use of intra-articular lidocaine as analgesia in anterior shoulder dislocation: a review and meta-analysis of the literature. Can J Rural Med. 2009;14(4):145–9.
Jiang N, Hu YJ, Zhang KR, Zhang S, Bin Y. Intra-articular lidocaine versus intravenous analgesia and sedation for manual closed reduction of acute anterior shoulder dislocation: an updated meta-analysis. J Clin Anesth. 2014;26(5):350–9.
Hames H, McLeod S, Millard W. Intra-articular lidocaine versus intravenous sedation for the reduction of anterior shoulder dislocations in the emergency department. CJEM. 2011;13(6):378–83.
American College of Emergency Physicians (ACEP) Managing acute pain in the ED: intra-articular posterior shoulder injection. 2021. https://www.acep.org/patient-care/map/map-intra-articular-posterior-shoulder-injection-tool/. Accessed 16 Sept 2021.
Kashani P, Zarchi FA, Hatamabadi HR, Afshar A, Amiri M. Intra-articular lidocaine versus intravenous sedative and analgesic for reduction of anterior shoulder dislocation. Turk J Emerg Med. 2016;16(2):60–4.
Koneri N, Georges N, Sirovich D, Slane M, Zitek T. Intra-articular lidocaine versus procedural sedation for anterior shoulder dislocations. Ann Emerg Med. 2020;76(4):S31.
Pradhan RL, Lakhey S, Pandey BK, Rijal KP. Reduction of acute anterior shoulder dislocations: comparing intraarticular lignocaine with intravenous anesthesia. J Nepal Med Assoc. 2006;45(162):223–7.
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. https://training.cochrane.org/handbook/current/chapter-08. Accessed 6 Feb 2021.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Cheok CY, Mohamad JA, Ahmad TS. Pain relief for reduction of acute anterior shoulder dislocations: a prospective randomized study comparing intravenous sedation with intra-articular lidocaine. J Orthop Trauma. 2011;25:5–10.
Sage W, Pickup L, Smith TO, et al. The clinical and functional outcomes of ultrasound-guided vs landmark-guided injections for adults with shoulder pathology—a systematic review and metaanalysis. Rheumatology. 2013;52(4):743–51.
Fitch RW, Kuhn JE. Intraarticular lidocaine versus intravenous procedural sedation with narcotics and benzodiazepines for reduction of the dislocated shoulder: a systematic review. Acad Emerg Med. 2008;15(8):703–8.
Wakai A, O’Sullivan R, McCabe A. Intra-articular lignocaine versus intravenous analgesia with or without sedation for manual reduction of acute anterior shoulder dislocation in adults. Cochrane Database Syst Rev. 2011;2011(4):CD004919.
Hunter B, Wilbur L. Can intra-articular lidocaine supplant the need for procedural sedation for reduction of acute anterior shoulder dislocation? Ann Emerg Med. 2012;59(6):513–4.
Penn M, Williams O. BET 1: can acute shoulder dislocations be reduced using intra-articular local anaesthetic infiltration as an alternative to intravenous analgesia with or without sedation? Emerg Med J. 2020;37(11):725–8.
Gould FJ. An effective treatment in the austere environment? A critical appraisal into the use of intra-articular local anesthetic to facilitate reduction in acute shoulder dislocation. Wilderness Environ Med. 2018;29(1):102–10.
Waterbrook AL, Paul S. Intra-articular lidocaine injection for shoulder reductions: a clinical review. Sports Health. 2011;3(6):556–9.
Tran VT, Ravaud P. Frugal innovation in medicine for low resource settings. BMC Med. 2016;14(1):1–3.
Nugus P, Holdgate A, Fry M, Forero R, McCarthy S, Braithwaite J. Work pressure and patient flow management in the emergency department: findings from an ethnographic study. Acad Emerg Med. 2011;18(10):1045–52.
Whiteside T, Kane E, Aljohani B, Alsamman M, Pourmand A. Redesigning emergency department operations amidst a viral pandemic. Am J Emerg Med. 2020;38(7):1448–53.
Schreyer KE, Daniel A, King LL, Blome A, DeAngelis M, Stauffer K, Desrochers K, Donahue W, Politarhos N, Raab C, McNamara R. Emergency department management of the COVID-19 pandemic. J Emerg Med. 2020;59(6):946–51.
Abdolrazaghnejad A, Banaie M, Tavakoli N, Safdari M, Rajabpour-Sanati A. Pain management in the emergency department: a review article on options and methods. Adv J Emerg Med. 2018;2(4):e45.
Symington L, Thakore S. A review of the use of propofol for procedural sedation in the emergency department. Emerg Med J. 2006;23(2):89–93.
Author information
Authors and Affiliations
Contributions
AS, SLM and KG conceived the study and designed the protocol. CW designed and executed the search strategy. AS and CT extracted the data and completed the risk of bias assessments. SLM conducted the meta-analysis. SLM and KG supervised the conduct of the study and data collection. AS and KG provided clinical advice on study interpretation. AS drafted the manuscript, and all authors contributed substantially to its revision. SLM takes responsibility for the paper as a whole.
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sithamparapillai, A., Grewal, K., Thompson, C. et al. Intra-articular lidocaine versus intravenous sedation for closed reduction of acute anterior shoulder dislocation in the emergency department: a systematic review and meta-analysis. Can J Emerg Med 24, 809–819 (2022). https://doi.org/10.1007/s43678-022-00368-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43678-022-00368-z