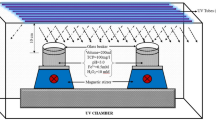
Abstract
Epoxiconazole (EPO) is classified as a persistent organic pollutant due to its ability to persist in the environment for prolonged periods. Its degradation is pivotal in mitigating its environmental impact. This investigation focuses on assessing the degradation of EPO using various methodologies, namely Fenton, photo-Fenton, solar photo-Fenton, and solar photolysis, conducted in both Milli-Q water and groundwater. These experiments encompassed evaluations at both the standard pH typically used in photo-Fenton reactions and the natural pH levels inherent to the respective aqueous environments. Additionally, EPO degradation products were analyzed after a 60-min reaction. Notably, in systems utilizing groundwater, the inclusion of additional iron was unnecessary, as the naturally occurring iron content in the groundwater facilitated the intended processes. Specifically, in Milli-Q water, solar photo-Fenton demonstrated an EPO degradation efficiency of 97%. Furthermore, the substitution of Milli-Q water with groundwater in Fenton-like processes did not significantly affect the efficacy of EPO degradation. These findings underscore the potential of solar photo-Fenton as an economically viable and environmentally sustainable strategy for EPO degradation.
Graphical abstract







Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Xue, P., Liu, X., Jia, H., Yuan, H., Liu, B., Zhang, J., & He, Z. (2022). Environmental behavior of the chiral fungicide epoxiconazole in earthworm-soil system: Enantioselective enrichment, degradation kinetics, chiral metabolite identification, and biotransformation mechanism. Environment International, 167, 107442. https://doi.org/10.1016/j.envint.2022.107442
Lv, X., Pan, L., Wang, J., Lu, L., Yan, W., Zhu, Y., Xu, Y., Guo, M., & Zhuang, S. (2017). Effects of triazole fungicides on androgenic disruption and CYP3A4 enzyme activity. Environmental Pollution, 222, 504–512. https://doi.org/10.1016/j.envpol.2016.11.051
Gleeson, T., Befus, K. M., Jasechko, S., Luijendijk, E., & Cardenas, M. B. (2016). The global volume and distribution of modern groundwater. Nature Geosci, 9, 161–167. https://doi.org/10.1038/ngeo2590
Kanakaraju, D., Glass, B. D., & Oelgemöller, M. (2018). Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. Journal of Environmental Management, 219, 189–207. https://doi.org/10.1016/j.jenvman.2018.04.103
Bartolomeu, M., Neves, M. G. P. M. S., Faustino, M. A. F., & Almeida, A. (2018). Wastewater chemical contaminants: remediation by advanced oxidation processes. Photochemical & Photobiological Sciences, 17, 1573–1598. https://doi.org/10.1039/C8PP00249E
Ahile, U. J., Wuana, R. A., Itodo, A. U., Sha’Ato, R., & Dantas, R. F. (2020). A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Science of The Total Environment, 710, 134872. https://doi.org/10.1016/j.scitotenv.2019.134872
Conte, L. O., Schenone, A. V., Giménez, B. N., & Alfano, O. M. (2019). Photo-Fenton degradation of a herbicide (2,4-d) in groundwater for conditions of natural pH and presence of inorganic anions. Journal of Hazardous Materials, 372, 113–120. https://doi.org/10.1016/j.jhazmat.2018.04.013
Reynoso, A., Zizzias, J., Sacchetto, J., Gambetta, C., Natera, J., & Massad, W. A. (2021). Complete benzothiazole elimination by the solar photo-Fenton process in aqueous and β-cyclodextrin solutions. New Journal of Chemistry, 45, 20214–20218. https://doi.org/10.1039/D1NJ02483C
Possetto, D., Natera, J., Sancho, M. I., García, N. A., & Massad, W. A. (2018). Bioallethrin degradation by photo-Fenton process in acetonitrile/water and aqueous β-cyclodextrin solutions. Journal of Photochemistry and Photobiology A: Chemistry, 365, 103–109. https://doi.org/10.1016/j.jphotochem.2018.07.036
Dong, H., Xu, L., Mao, Y., Wang, Y., Duan, S., Lian, J., Li, J., Yu, J., & Qiang, Z. (2021). Effective abatement of 29 pesticides in full-scale advanced treatment processes of drinking water: From concentration to human exposure risk. Journal of Hazardous Materials, 403, 123986. https://doi.org/10.1016/j.jhazmat.2020.123986
Murthy, N. B. K., Hustert, K., Moza, P. N., & Kettrup, A. (1998). Photodegradation of selected fungicides on soil. Fresenius Environmental Bulletin, 7, 112–117.
Pacholak, A., Burlaga, N., Frankowski, R., Zgoła-Grześkowiak, A., & Kaczorek, E. (2022). Azole fungicides: (Bio)degradation, transformation products and toxicity elucidation. Science of The Total Environment, 802, 149917. https://doi.org/10.1016/j.scitotenv.2021.149917
Bryant, M., & Latimer, G. W. (Eds.). (2023). Water. Oxford University Press. https://doi.org/10.1093/9780197610145.003.078
2130 Turbidity. (2017). In Standard Methods For the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater. American Public Health Association. https://doi.org/10.2105/SMWW.2882.018
4500-NO3--nitrogen (nitrate) (2017) In Standard Methods For the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater. American Public Health Association. https://doi.org/10.2105/SMWW.2882.089
2320 Alkalinity (2017) In Standard Methods For the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater. American Public Health Association. https://doi.org/10.2105/SMWW.2882.023
3125 Metals by Inductively Coupled Plasma-Mass Spectrometry (2017) In Standard Methods For the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater. American Public Health Association.
Nogueira, R. F. P., Oliveira, M. C., & Paterlini, W. C. (2005). Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate. Talanta, 66, 86–91. https://doi.org/10.1016/j.talanta.2004.10.001
Subramanian, G., & Madras, G. (2016). Introducing saccharic acid as an efficient iron chelate to enhance photo-Fenton degradation of organic contaminants. Water Research, 104, 168–177. https://doi.org/10.1016/j.watres.2016.07.070
Konášová, R., Dytrtová, J. J., & Kašička, V. (2015). Determination of acid dissociation constants of triazole fungicides by pressure assisted capillary electrophoresis. Journal of Chromatography A, 1408, 243–249. https://doi.org/10.1016/j.chroma.2015.07.005
Mirzaei, A., Chen, Z., Haghighat, F., & Yerushalmi, L. (2017). Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere, 174, 665–688. https://doi.org/10.1016/j.chemosphere.2017.02.019
Fischbacher, A., von Sonntag, C., & Schmidt, T. C. (2017). Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe(II) ratios. Chemosphere, 182, 738–744. https://doi.org/10.1016/j.chemosphere.2017.05.039
Giménez, B. N., Conte, L. O., Alfano, O. M., & Schenone, A. V. (2020). Paracetamol removal by photo-Fenton processes at near-neutral pH using a solar simulator: Optimization by D-optimal experimental design and toxicity evaluation. Journal of Photochemistry and Photobiology A: Chemistry, 397, 112584. https://doi.org/10.1016/j.jphotochem.2020.112584
Liu, C., Qiang, Z., Tian, F., & Zhang, T. (2009). Photodegradation of etridiazole by UV radiation during drinking water treatment. Chemosphere, 76, 609–615. https://doi.org/10.1016/j.chemosphere.2009.04.052
Burrows, H. D., Canle, L. M., Santaballa, J. A., & Steenken, S. (2002). Reaction pathways and mechanisms of photodegradation of pesticides. Journal of Photochemistry and Photobiology B: Biology, 67, 71–108. https://doi.org/10.1016/S1011-1344(02)00277-4
Rodríguez-Cabo, T., Rodríguez, I., Ramil, M., & Cela, R. (2018). Evaluation of the aqueous phototransformation routes of phenyl ethyl azolic fungicides by liquid chromatography accurate mass spectrometry. Science of The Total Environment, 615, 942–954. https://doi.org/10.1016/j.scitotenv.2017.10.003
Kavitha, V., & Palanivelu, K. (2004). The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere, 55, 1235–1243. https://doi.org/10.1016/j.chemosphere.2003.12.022
Buxton, G. V., Greenstock, C. L., Helman, W. P., & Ross, A. B. (1988). Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/O− in Aqueous Solution. Journal of Physical and Chemical Reference Data, 17, 513–886. https://doi.org/10.1063/1.555805
Gligorovski, S., Strekowski, R., Barbati, S., & Vione, D. (2015). Environmental Implications of Hydroxyl Radicals (·OH). Chemical Reviews, 115, 13051–13092. https://doi.org/10.1021/cr500310b
Mack, J., & Bolton, J. R. (1999). Photochemistry of nitrite and nitrate in aqueous solution: A review. Journal of Photochemistry and Photobiology A: Chemistry, 128, 1–13. https://doi.org/10.1016/S1010-6030(99)00155-0
Zhou, H., Lian, L., Yan, S., & Song, W. (2017). Insights into the photo-induced formation of reactive intermediates from effluent organic matter: The role of chemical constituents. Water Research, 112, 120–128. https://doi.org/10.1016/j.watres.2017.01.048
Rodríguez-López, L., Cela-Dablanca, R., Núñez-Delgado, A., Álvarez-Rodríguez, E., Fernández-Calviño, D., & Arias-Estévez, M. (2021). Photodegradation of ciprofloxacin, clarithromycin and trimethoprim: Influence of pH and humic acids. Molecules, 26, 3080.
Acknowledgements
The authors express our gratitude to the Secretaría de Ciencia y Técnica of Universidad Nacional de Río Cuarto (SECyT-UNRC), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), all based in Argentina, for their generous financial support. In addition, the authors would like to thank JLA Argentina S.A. for allowing the use of their facilities and equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sacchetto, J.L., Medina, L.F., Toledo, K.I. et al. Epoxiconazole degradation in water samples: a comparative study of Fenton, photo-Fenton, solar photo-Fenton, and solar photolysis processes. Photochem Photobiol Sci (2024). https://doi.org/10.1007/s43630-024-00582-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43630-024-00582-x