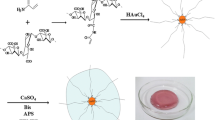
Abstract
Nanosized alginate-based particles (NAPs) were obtained in a one-pot solvent-free synthesis procedure, achieving the design of a biocompatible nanocarrier for the encapsulation of IbM6 antimicrobial peptide (IbM6). IbM6 is integrated in the nascent nanosized hydrogel self-assembly guided by electrostatic interactions and by weak interactions, typical of soft matter. The formation of the nanogel is a dynamic and complex process, which presents an interesting temporal evolution. In this work, we optimized the synthesis conditions of IbM6-NAPs based on small-angle X-ray scattering (SAXS) measurements and evaluated its time evolution over several weeks by sensing the IbM6 environment in IbM6-NAPs from photochemical experiments. Fluorescence deactivation experiments revealed that the accessibility of different quenchers to the IbM6 peptide embedded in NAPs is dependent on the aging time of the alginate network. Lifetimes measurements indicate that the deactivation paths of the excited state of the IbM6 in the nanoaggregates are reduced when compared with those exhibited by the peptide in aqueous solution, and are also dependent on the aging time of the nanosized alginate network. Finally, the entrapment of IbM6 in NAPs hinders the degradation of the peptide by trypsin, increasing its antimicrobial activity against Escherichia coli K-12 in simulated operation conditions.
Graphical abstract





Similar content being viewed by others
References
WHO Geneva. (2017). Global action plan on antimicrobial resistance. World Health Organization, 1–28.
Santos, M. A. D. O., Vianna, M. F., Nishino, L. K., & Lazarini, P. R. (2015). Vestibular disorders in Bell’s palsy: A prospective study. Revue de Laryngologie Otologie Rhinologie (Bord), 136(1), 29–31.
Larsson, D. G. J., & Flach, C. F. (2022). Antibiotic resistance in the environment. Nature Reviews Microbiology, 20(5), 257–269. https://doi.org/10.1038/s41579-021-00649-x
Szymczak, P., et al. (2023). Discovering highly potent antimicrobial peptides with deep generative model HydrAMP. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-36994-z.
Li, C., et al. (2022). AMPlify: Attentive deep learning model for discovery of novel antimicrobial peptides effective against WHO priority pathogens. BMC Genomics, 23(1), 1–15. https://doi.org/10.1186/s12864-022-08310-4
Marr, A. K., Gooderham, W. J., & Hancock, R. E. W. (2006). Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Current Opinion in Pharmacology, 6, 468–472. https://doi.org/10.1016/j.coph.2006.04.006
Wiman, E., Zattarin, E., Aili, D., Bengtsson, T., & Selegård, R. (2023). Development of novel broad – spectrum antimicrobial lipopeptides derived from plantaricin NC8 β. Science and Reports, 0123456789, 1–16. https://doi.org/10.1038/s41598-023-31185-8
Klubthawee, N., Adisakwattana, P., Hanpithakpong, W., Somsri, S., & Aunpad, R. (2020). A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. Science and Reports, 10(1), 1–17. https://doi.org/10.1038/s41598-020-65688-5
Bulet, P. (2004). Anti-microbial peptides: From invertebrates to vertebrates. Immunological Reviews, 198, 169–184.
Flórez-Castillo, J. M., Perullini, M., Jobbágy, M., & De Jesús Cano Calle, H. (2014). Enhancing antibacterial activity against Escherichia coli K-12 of peptide Ib-AMP4 with synthetic analogues. International Journal of Peptide Research and Therapeutic, 20(3). https://doi.org/10.1007/s10989-014-9391-2.
Flórez-Castillo, J. M., et al. (2020). Ib-M6 antimicrobial peptide: Antibacterial activity against clinical isolates of Escherichia coli and molecular docking. Antibiotics, 9(2). https://doi.org/10.3390/antibiotics9020079.
Zhang, Q. Y., et al. (2021). Antimicrobial peptides: Mechanism of action, activity and clinical potential. Military Medical Research, 8(1), 1–25. https://doi.org/10.1186/s40779-021-00343-2
Rowlett, V. W., et al. (2017). Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. Journal of Bacteriology, 199(13). https://doi.org/10.1128/JB.00849-16.
Huang, Y. T., Kumar, S. R., Chan, H. C., Jhan, Z. H., Chen, D. W., & Lue, S. J. (2021). Efficacy of antimicrobial peptides (AMPs) against Escherichia coli and bacteria morphology change after AMP exposure. Journal of the Taiwan Institute of Chemical Engineers, 126, 307–312. https://doi.org/10.1016/j.jtice.2021.07.003
El-Feky, G. S., El-Banna, S. T., El-Bahy, G. S., Abdelrazek, E. M., & Kamal, M. (2017). Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydrate Polymers, 177(January), 194–202. https://doi.org/10.1016/j.carbpol.2017.08.104
Rodrigues da Silva, G. H., et al. (2020). Injectable in situ forming nanogel: A hybrid Alginate-NLC formulation extends bupivacaine anesthetic effect”. Materials Science and Engineering C, 109, 110608. https://doi.org/10.1016/j.msec.2019.110608
Venkatesan, J., Bhatnagar, I., Manivasagan, P., Kang, K. H., & Kim, S. K. (2015). Alginate composites for bone tissue engineering: A review. International Journal of Biological Macromolecules, 72, 269–281. https://doi.org/10.1016/j.ijbiomac.2014.07.008
Sonego, J. M., Santagapita, P. R., Perullini, M., & Jobbágy, M. (2016). Ca(II) and Ce(III) homogeneous alginate hydrogels from the parent alginic acid precursor: A structural study. Dalton Transactions, 45(24). https://doi.org/10.1039/c6dt00321d.
Posbeyikian, A., et al. (2021). Evaluation of calcium alginate bead formation kinetics: An integrated analysis through light microscopy, rheology and microstructural SAXS. Carbohydrate Polymers, 269(February), 1–10. https://doi.org/10.1016/j.carbpol.2021.118293
Ingar, K., & Skja, G. (2006). Similarities and differences between alginic acid gels and ionically crosslinked alginate gels. Food Hydrocolloids, 20, 170–175. https://doi.org/10.1016/j.foodhyd.2004.03.009
Spedalieri, C., et al. (2015). Silica@proton-alginate microreactors: A versatile platform for cell encapsulation. Journal of Materials Chemistry B, 3(16), 2015. https://doi.org/10.1039/c4tb02020k
Osorio-Alvarado, C. E., Ropero-Vega, J. L., Farfán-García, A. E., & Flórez-Castillo, J. M. (2022). Immobilization systems of antimicrobial peptide Ib−M1 in polymeric nanoparticles based on alginate and chitosan. Polymers (Basel), 14(15), 1–14. https://doi.org/10.3390/polym14153149
Alkhatib, H., Mohamed, F., Akkawi, M. E., Alfatama, M., Chatterjee, B., & Doolaanea, A. A. (2020). Microencapsulation of black seed oil in alginate beads for stability and taste masking. Journal of Drug Delivery Science and Technology, 60(August), 102030. https://doi.org/10.1016/j.jddst.2020.102030.
Perullini, M., Ferro, Y., Durrieu, C., Jobbágy, M., & Bilmes, S. A. (2014). Sol-gel silica platforms for microalgae-based optical biosensors. Journal of Biotechnology, 179(1). https://doi.org/10.1016/j.jbiotec.2014.02.007.
Guarino, V., Altobelli, R., della Sala, F., Borzacchiello, A., & Ambrosio, L. (2018). Alginate processing routes to fabricate bioinspired platforms for tissue engineering and drug delivery. Springer Series in Biomaterials Science and Engineering, 11, 101–120. https://doi.org/10.1007/978-981-10-6910-9_4.
Zazzali, I., et al. (2022). Fine-tuning of functional and structural properties of Ca(II)-alginate beads containing artichoke waste extracts. Food Hydrocolloids for Health, 2(August). https://doi.org/10.1016/j.fhfh.2022.100097.
Paques, J. P., Van Der Linden, E., Van Rijn, C. J. M., & Sagis, L. M. C. (2014). Preparation methods of alginate nanoparticles. Advances in Colloid and Interface Science, 209, 163–171. https://doi.org/10.1016/j.cis.2014.03.009
Hamidi, M., Azadi, A., & Rafiei, P. (2008). Hydrogel nanoparticles in drug delivery. Advanced Drug Delivery Reviews, 60(15), 1638–1649. https://doi.org/10.1016/j.addr.2008.08.002
Flórez-Castillo, J. M., Ropero-Vega, J. L., Perullini, M., & Jobbágy, M. (2019). Biopolymeric pellets of polyvinyl alcohol and alginate for the encapsulation of Ib-M6 peptide and its antimicrobial activity against E. coli. Heliyon, 5(6). https://doi.org/10.1016/j.heliyon.2019.e01872.
Ropero-Vega, J. L., Ardila-Rosas, N., Hernández, I. P., & Flórez-Castillo, J. M. (2020). Immobilization of Ib-M2 peptide on core@shell nanostructures based on SPION nanoparticles and their antibacterial activity against Escherichia coli O157:H7. Applied Surface Science, 515(March), 146045. https://doi.org/10.1016/j.apsusc.2020.146045.
Salvati, B., Santagapita, P., & Perullini, M. (2022). Exploring the conditions to generate alginate nanogels. Journal of Sol-Gel Science and Technology, 102, 142–150.
Aguirre Calvo, T. R., Santagapita, P. R., & Perullini, M. (2019). Functional and structural effects of hydrocolloids on Ca(II)-alginate beads containing bioactive compounds extracted from beetroot. LWT, 111, 520–526. https://doi.org/10.1016/j.lwt.2019.05.047
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 2, 248–254. https://doi.org/10.1016/j.cj.2017.04.003
Traffano-Schiffo, M. V., Castro-Giraldez, M., Fito, P. J., Perullini, M., & Santagapita, P. R. (2018). Gums induced microstructure stability in Ca(II)-alginate beads containing lactase analyzed by SAXS. Carbohydrate Polymers, 179, 402–407. https://doi.org/10.1016/j.carbpol.2017.09.096
Eftinkt, M. R., & Ghiron, C. A. (1977). Exposure of tryptophanyl residues and protein dynamics. Biochemistry, 16(25), 5546–5551. https://doi.org/10.1021/bi00644a024
Albani, J. R. (2014). Origin of tryptophan fluorescence lifetimes. Part 2: Fluorescence lifetimes origin of tryptophan in proteins. Journal of Fluorescence, 24(1), 105–117. https://doi.org/10.1007/s10895-013-1274-y
Valeur, B. (2002). Molecular fluorescence: Principles and applications. Wiley.
Gardiner, C. W. (1990). Handbook of stochastic methods for physics, chemistry, and the natural sciences (2nd ed.). Springer.
Eftink, M. R., & Ghiron, C. A. (1976). Fluorescence quenching of indole and model micelle systems. Journal of Physical Chemistry, 80(5), 486–493.
Szabo, A. G., & Rayner, D. M. (1980). Fluorescence decay of tryptophan conformers in aqueous solution. Journal of the American Chemical Society, 102(2), 554–563. https://doi.org/10.1021/ja00522a020
Lakowicz, J. R. (2006). Principles of fluorescence spectroscopy. Springer.
Acknowledgements
This work was supported by funds from the Faculty of Exact and Natural Sciences of the University of Buenos Aires (FCEyN, UBA, +4i project), the National Scientific and Technological Research Council of Argentina (CONICET), PIP 11220170100991CO and the Brazilian Synchrotron Light Laboratory (LNLS, Brazil, proposal SAXS1-20190143 and SAXS1-20190073). We acknowledge the technical assistance of Luciana Pavone in part of the experimental work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salvati, B., Flórez-Castillo, J.M., Santagapita, P.R. et al. One-pot synthesis of alginate-antimicrobial peptide nanogel. Photochem Photobiol Sci 23, 665–679 (2024). https://doi.org/10.1007/s43630-024-00542-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-024-00542-5