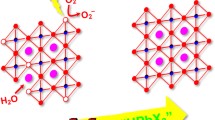
Abstract
Inorganic halide perovskites, such as CsPbI3, have unique optoelectronic properties which made them promising candidates for several applications. Unfortunately, these perovskites undergo rapid chemical decomposition and transformation into yellow δ-phase. Thus, the synthesis of stable cesium lead iodide perovskites remains an actual challenging field and it is imperative to develop a stabilized black phase for photovoltaic applications. For this purpose, a surfactant ligand was used to control the synthesis of inorganic perovskite CsPbI3 nanoparticles. Herein we demonstrate a new avenue for lead halide perovskites with the addition of either hexadecyltrimethylammonium bromide (CTAB) or silica nanoparticles to maintain in the first place; the stability of the α-CsPbI3 phase, and later on to boost their photoluminescence quantum yield (PLQY). The prepared perovskites were characterized using UV–visible absorption spectroscopy, fluorescence spectroscopy, scanning electron microscopy, thermogravimetric analysis and X-Ray diffraction technique. Results show higher stability of α-CsPbI3 phase and improvement in PLQY % to reach 99% enhancement in presence of CTAB. Moreover, the photoluminescence intensity of CsPbI3 nanoparticles was higher and was maintained for a longer duration in the presence of CTAB.
Graphical abstract









Similar content being viewed by others
References
Atta, N. F., Galal, A., & El-Ads, E. H. (2016). Perovskite nanomaterials—Synthesis, characterization, and applications. In L. Pan, G. Zhu (Eds.), Perovskite Materials-Synthesis, Characterisation, Properties, and Applications (pp. 107–152). Rijeka, Croata: InTech.
Dimitrovska, S., Aleksovska, S., & Kuzmanovski, I. (2005). Prediction of the unit cell edge length of cubic A22+BB′O6 perovskites by multiple linear regression and artificial neural networks. Central European Journal of Chemistry, 3(1), 198–215.
Snaith, H. J. (2013). Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. Journal of Physical Chemistry Letters, 4(21), 3623–3630.
Manseki, K., Ikeya, T., Tamura, A., Ban, T., Sugiura, T., & Yoshida, T. (2014). Mg-doped TiO2 nanorods improving open-circuit voltages of ammonium lead halide perovskite solar cells. RSC Advances, 4(19), 9652–9655.
Kim, H.-S., Lee, C.-R., Im, J.-H., Lee, K.-B., Moehl, T., Marchioro, A., Moon, S.-J., Humphry-Baker, R., Yum, J.-H., Moser, J. E., Grätzel, M., & Park, N.-G. (2012). Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Scientific Reports, 2, 1–7.
Stranks, S. D., & Snaith, H. J. (2015). Metal-halide perovskites for photovoltaic and light-emitting devices. Nature Nanotechnology, 10(5), 391–402.
Petrović, M., Chellappan, V., & Ramakrishna, S. (2015). Perovskites: Solar cells & engineering applications—Materials and device developments. Solar Energy, 122, 678–699.
Wang, J., Shi, S. Q., Chen, L. Q., Li, Y., & Zhang, T. Y. (2004). Phase-field simulations of ferroelectric/ferroelastic polarization switching. Acta Materialia, 52(3), 749–764.
Spaldin, N. A., Cheong, S. W., & Ramesh, R. (2010). Multiferroics: Past, present, and future. Physics Today, 63(10), 38–43.
Wang, C., Jin, K., Zhao, R., Lu, H., Guo, H., Ge, C., He, M., Wang, C., & Yang, G. (2011). Ultimate photovoltage in perovskite oxide heterostructures with critical film thickness. Applied Physics Letters, 98(18), 2009–2012.
Nieto, S., Polanco, R., & Roque-Malherbe, R. (2007). Absorption kinetics of hydrogen in nanocrystals of BaCe 0.95Yb0.05O3-δ proton-conducting perovskite. Journal of Physical Chemistry C, 111(6), 2809–2818.
Mankiewich, P. M., Scofield, J. H., Skocpol, W. J., Howard, R. E., Dayem, A. H., & Good, E. (1987). Reproducible technique for fabrication of thin films of high transition temperature superconductors. Applied Physics Letters, 51(21), 1753–1755.
Pottathara, Y. B., & Thomas, S. (2019). Synthesis and processing of emerging two-dimensional nanomaterials. Amsterdam: ScienceDirect.
Nakamura, T., Misono, M., & Yoneda, Y. (1983). Reduction–oxidation and catalytic properties of La1 - xSrxCoO3. Journal of Catalysis, 83(1), 151–159.
Esposito, S. (2019). ‘Traditional’ sol–gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials (Basel), 12(4), 1–25.
Kemnitz, E., & Noack, J. (2015). The non-aqueous fluorolytic sol–gel synthesis of nanoscaled metal fluorides. The Royal Society of Chemistry, 44, 19411–19431.
Lozano-Gorrin, A. D. (2012). Structural characterization of new perovskites (pp. 107–204). London: Intech.
Wang, Q., Zheng, X., Deng, Y., Zhao, J., Chen, Z., & Huang, J. (2017). Stabilizing the α-phase of CsPbI3 perovskite by sulfobetaine zwitterions in one-step spin-coating films. Joule, 1(2), 371–382.
Marronnier, A., Roma, G., Boyer-Richard, S., Pedesseau, L., Jancu, J. M., Bonnassieux, Y., Katan, C., Stoumpos, C. C., Kanatzidis, M. G., & Even, J. (2018). Anharmonicity and disorder in the black phases of CsPbI3 used for stable inorganic perovskite solar cells. American Chemical Society, 3, 1715–1717.
Zhou, Y., & Yixin, Z. (2019). Chemical stability and instability of inorganic halide perovskites. Energy & Environmental Science, 12, 1495–1511.
Protesescu, L., Yakunin, S., Bodnarchuk, M. I., Krieg, F., Caputo, R., Hendon, C. H., Yang, R. X., Walsh, A., & Kovalenko, M. V. (2015). Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Letters, 6, 3692–3696.
Dastidar, S., Egger, D. A., Tan, L. Z., Cromer, S. B., Dillon, A. D., Liu, S., Kronik, L., Rappe, A. M., & Fafarman, A. T. (2016). High chloride doping levels stabilize the perovskite phase of cesium lead iodide. Nano Letters, 16(6), 3563–3570.
Cai, Y., Zhang, P., Bai, W., Lu, L., Wang, L., Chen, X., & Xie, R. J. (2022). Synthesizing bright CsPbBr3 perovskite nanocrystals with high purification yields and their composites with in situ-polymerized styrene for light-emitting diode applications. ACS Sustainable Chemistry & Engineering, 10(22), 7385–7393.
Rao, K. S., El-Hami, K., Kodaki, T., Matsushige, K., & Makino, K. (2005). A novel method for synthesis of silica nanoparticles. Journal of Colloid and Interface Science, 289(1), 125–131.
Murphy, J. P. (2018). Novel hybrid perovskite composites and microstructures: Synthesis and characterization (pp. 1–110). Mont. Tech Libr., no. June.
Hu, Y., Bai, F., Liu, X., Ji, Q., Miao, X., Qiu, T., & Zhang, S. (2017). Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. American Chemical Society, 2(10), 2219–2227.
Protesescu, L., Yakunin, S., Bodnarchuk, M. I., Krieg, F., Caputo, R., Hendon, C. H., Yang, R. X., Walsh, A., & Kovalenko, M. V. (2015). Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Letters, 15(6), 3692–3696.
Wu, X., Trinh, M. T., Niesner, D., Zhu, H., Norman, Z., Owen, J. S., Yaffe, O., Kudisch, B. J., & Zhu, X. Y. (2015). Trap states in lead iodide perovskites. Journal of the American Chemical Society, 137(5), 2089–2096.
Eze, V. O., Lei, B., & Mori, T. (2016). Air-assisted flow and two-step spin-coating for highly efficient CH3NH3PbI3 perovskite solar cells. Japanese Journal of Applied Physics, 55(2), 2–8.
Kumar, M., Pawar, V., Jha, P. A., Gupta, S. K., Sinha, A. S., Jha, P. K., & Singh, P. (2019). Thermo-optical correlation for room temperature synthesis: cold-sintered lead halides. Journal of Materials Science: Materials in Electronics, 30(6), 6071–6081.
Aleksanyan, E., Aprahamian, A., Mukasyan, A. S., Harutyunyan, V., & Manukyan, K. V. (2020). Mechanisms of mechanochemical synthesis of cesium lead halides: Pathways toward stabilization of α-CsPbI3. Journal of Materials Science, 55(20), 8665–8678.
Miyasaka, T., Kulkarni, A., Kim, G. M., Öz, S., & Jena, A. K. (2020). Perovskite solar cells: can we go organic-free, lead-free, and dopant-free? Advanced Energy Materials, 10(13), 1902500.
Monshi, A., Foroughi, M. R., & Monshi, M. R. (2012). Modified scherrer equation to estimate more accurately nano-crystallite size using XRD. World Journal of Nano Science and Engineering, 2(3), 154–160.
Boote, B. W., Andaraarachchi, H. P., Rosales, B. A., Blome-Fernández, R., Zhu, F., Reichert, M. D., Santra, K., Li, J., Petrich, J. W., Vela, J., & Smith, E. A. (2019). Unveiling the photo- and thermal-stability of cesium lead halide perovskite nanocrystals. ChemPhysChem, 20(20), 2647–2656.
Cho, N.-K., Na, H.-J., Yoo, J., & Kim, Y. S. (2021). Long-term stability in γ-CsPbI3 perovskite via an ultraviolet-curable polymer network. Communications Materials, 2(1), 1–8.
Ji, Y., Zhang, J. B., Shen, H. R., Su, Z., Cui, H., Lan, T., Wang, J. Q., Chen, Y. H., Liu, L., Cao, K., & Shen, W. (2021). Improving the stability of α-CsPbI3 nanocrystals in extreme conditions facilitated by Mn2+ doping. ACS Omega, 6(21), 13831–13838.
Motta, C., El-Mellouhi, F., Kais, S., Tabet, N., Alharbi, F., & Sanvito, S. (2015). Revealing the role of organic cations in hybrid halide perovskite CH3 NH3PbI3. Nature Communications, 6, 1–7.
Ma, J., & Wang, L. W. (2015). Nanoscale charge localization induced by random orientations of organic molecules in hybrid perovskite CH3NH3PbI3. Nano Letters, 15(1), 248–253.
Zhou, Y., Huang, F., Cheng, Y. B., & Gray-Weale, A. (2015). Photovoltaic performance and the energy landscape of CH3NH3PbI3. Physical Chemistry Chemical Physics: PCCP, 17(35), 22604–22615.
Mosconi, E., Meggiolaro, D., Snaith, H. J., Stranks, S. D., & De Angelis, F. (2016). Light-induced annihilation of Frenkel defects in organo-lead halide perovskites. Energy & Environmental Science, 9(10), 3180–3187.
Ghorai, A., Midya, A., & Ray, S. K. (2019). Surfactant-induced anion exchange and morphological evolution for composition-controlled caesium lead halide perovskites with tunable optical properties. ACS Omega, 4(7), 12948–12954.
Resch-Genger, U., & Rurack, K. (2013). Determination of the photoluminescence quantum yield of dilute dye solutions. Pure and Applied Chemistry, 85(10), 2005–2026.
Rene-Boisneuf, L., & Scaiano, J. C. (2008). Sensitivity versus stability: Making quantum dots more luminescent by sulfur photocuring without compromising sensor response. Chemistry of Materials, 20(21), 6638–6642.
Resch-Genger, U., & Rurack, K. (2013). Determination of the photoluminescence quantum yield of dilute dye solutions (IUPAC Technical Report). Pure and Applied Chemistry, 85(10), 2005–2026.
Bi, C., Kershaw, S. V., Rogach, A. L., & Tian, J. (2019). Improved stability and photodetector performance of CsPbI3 perovskite quantum dots by ligand exchange with aminoethanethiol. Advanced Functional Materials, 29(29), 1–9.
Zhang, L., Kang, C., Zhang, G., Pan, Z., Huang, Z., Xu, S., Rao, H., Liu, H., Wu, S., Wu, X., & Li, X. (2021). All-inorganic CsPbI3 quantum dot solar cells with efficiency over 16% by defect control. Advanced Functional Materials, 31(4), 1–10.
Shao, Y., Zhang, C., Zhou, C., Wang, T., Chen, J., Liu, X., Lin, J., & Chen, X. (2022). Designable and highly stable emissive CsPbI3 perovskite quantum dots/polyvinylidene fluoride nanofiber composites. Optical Materials Express, 12(1), 109.
Chen, L. C., Chang, Y. T., Tien, C. H., Yeh, Y. C., Tseng, Z. L., Lee, K. L., & Kuo, H. C. (2020). Red light-emitting diodes with all-inorganic CsPbI3/TOPO composite nanowires color conversion films. Nanoscale Research Letters, 15(1), 9.
Acknowledgements
Financial support provided by American University of Beirut, Lebanon through University Research Board (URB) as well as Kamal A. Shair Central Research Laboratory (KAS CRSL) facilities to carry out this work is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Tawil, C., El Kurdi, R. & Patra, D. Higher stability and better photoluminescence quantum yield of cesium lead iodide perovskites nanoparticles in the presence of CTAB ligand. Photochem Photobiol Sci 22, 2167–2178 (2023). https://doi.org/10.1007/s43630-023-00439-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00439-9