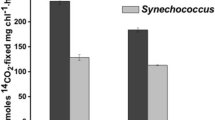
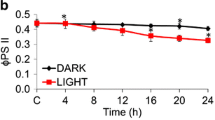
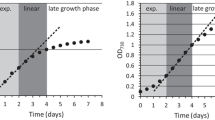
Abstract
Cyanobacteria are challenged by daily fluctuations of light intensities and photoperiod in their natural habitats, which affect the physiology and fitness of cyanobacteria. Circadian rhythms (CRs), an important endogenous process found in all organisms including cyanobacteria, control their physiological activities and helps in coping with 24-h light/dark (LD) cycle. In cyanobacteria, physiological responses under rhythmic ultraviolet radiation (UVR) are poorly studied. Therefore, we studied the changes in photosynthetic pigments, and physiological parameters of Synechocystis sp. PCC 6803 under UVR and photosynthetically active radiation (PAR) of light/dark (LD) oscillations having the combinations of 0, 4:20, 8:16, 12:12, 16:8, 20:4, and 24:24 h. The LD 16:8 enhanced the growth, pigments, proteins, photosynthetic efficiency, and physiology of Synechocystis sp. PCC6803. Continuous light (LL 24) of UVR and PAR exerted negative impact on the photosynthetic pigments, and chlorophyll fluorescence. Significant increase in reactive oxygen species (ROS) resulted in loss of plasma membrane integrity followed by decreased viability of cells. The dark phase played a significant role in Synechocystis to withstand the LL 24 under PAR and UVR. This study offers detailed understanding of the physiological responses of the cyanobacterium to changing light environment.
Graphical abstract









Similar content being viewed by others
Data availability
Data will be made avaialable from the authors on request.
References
Iwasaki, H., & Kondo, T. (2000). The current state and problems of circadian clock studies in cyanobacteria. Plant and Cell Physiology, 41(9), 1013–1020. https://doi.org/10.1093/pcp/pcd024
Reppert, S. M., & Weaver, D. R. (2002). Coordination of circadian timing in mammals. Nature, 418(6901), 935–941. https://doi.org/10.1038/nature00965
Dibner, C., Schibler, U., & Albrecht, U. (2010). The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annual Review of Physiology, 72, 517–549. https://doi.org/10.1146/annurev-physiol-021909-135821
Cohen, S. E., & Golden, S. S. (2015). Circadian rhythms in cyanobacteria. Microbiology and Molecular Biology Reviews, 79(4), 373–385. https://doi.org/10.1128/MMBR.00036-15
Liu, Y., Tsinoremas, N. F., Johnson, C. H., Lebedeva, N. V., Golden, S. S., Ishiura, M., & Kondo, T. (1995). Circadian orchestration of gene expression in cyanobacteria. Genes and Development, 9(12), 1469–1478. https://doi.org/10.1101/gad.9.12.1469
Mori, T., Binder, B., & Johnson, C. H. (1996). Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proceeding of the National Academy of Sciences USA, 93(19), 10183–10188. https://doi.org/10.1073/pnas.93.19.10183
Smith, R. M., & Williams, S. B. (2006). Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus. Proceeding of the National Academy of Sciences USA, 103(22), 8564–8569. https://doi.org/10.1073/pnas.0508696103
Diamond, S., Jun, D., Rubin, B. E., & Golden, S. S. (2015). The circadian oscillator in Synechococcus elongatus controls metabolite partitioning during diurnal growth. Proceeding of the National Academy of Sciences USA, 112(15), E1916–E1925. https://doi.org/10.1073/pnas.1504576112
Kumar, D., Kannaujiya, V. K., Pathak, J., Sundaram, S., & Sinha, R. P. (2018). Composition and functional property of photosynthetic pigments under circadian rhythm in the cyanobacterium Spirulina platensis. Protoplasma, 255(3), 885–898. https://doi.org/10.1007/s00709-017-1195-8
Kono, M., & Terashima, I. (2014). Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. Journal of Photochemistry Photobiology B: Biology, 137, 89–99. https://doi.org/10.1016/j.jphotobiol.2014.02.016
Cassier-Chauvat, C., Veaudor, T., & Chauvat, F. (2016). Comparative genomics of DNA recombination and repair in cyanobacteria: Biotechnological implications. Frontiers in Microbiology, 7, 1809. https://doi.org/10.3389/fmicb.2016.01809
Häder, D.-P., Williamson, C. E., Wängberg, S. Å., Rautio, M., Rose, K. C., Gao, K., Helbling, E. W., Sinha, R. P., & Worrest, R. (2015). Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochemistry and Photobiological Sciences, 14(1), 108–126. https://doi.org/10.1039/c4pp90035a
Sancar, A. (2016). Mechanisms of DNA repair by photolyase and excision nuclease (Nobel Lecture). Angewandte Chemie International Edition, 55(30), 8502–8527. https://doi.org/10.1002/anie.201601524
Singh, S. P., Häder, D.-P., & Sinha, R. P. (2010). Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Research Reviews, 9(2), 79–90. https://doi.org/10.1016/j.arr.2009.05.004
Pathak, J., Rajneesh., Singh, P. R., Häder, D.-P., & Sinha, R. P. (2019). UV-induced DNA damage and repair: A cyanobacterial perspective. Plant Gene, 19, 100194. https://doi.org/10.1016/j.plgene.2019.100194
Rajneesh., Pathak, J., Häder, D.-P., & Sinha, R. P. (2019). Impacts of ultraviolet radiation on certain physiological and biochemical processes in cyanobacteria inhabiting diverse habitats. Environmental and Experimental Botany, 161, 375–387. https://doi.org/10.1016/j.envexpbot.2018.10.037
Noyma, N. P., Silva, T. P., Chiarini-Garcia, H., Amado, A. M., Roland, F., & Melo, R. C. (2015). Potential effects of UV radiation on photosynthetic structures of the bloom-forming cyanobacterium Cylindrospermopsis raciborskii CYRF-01. Frontier in Microbiology, 6, 1202. https://doi.org/10.3389/fmicb.2015.01202
Lesser, M. P. (2008). Effects of ultraviolet radiation on productivity and nitrogen fixation in the cyanobacterium, Anabaena sp. (Newton’s strain). Hydrobiologia, 598(1), 1–9. https://doi.org/10.1007/s10750-007-9126-x
Singh, J. S., Kumar, A., Rai, A. N., & Singh, D. P. (2016). Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Frontiers in Microbiology, 7, 529. https://doi.org/10.3389/fmicb.2016.00529
Ahmed, H., Pathak, J., Sonkar, P. K., Ganesan, V., Häder, D.-P., & Sinha, R. P. (2021). Responses of a hot spring cyanobacterium under ultraviolet and photosynthetically active radiation: photosynthetic performance, antioxidative enzymes, mycosporine-like amino acid profiling and its antioxidative potentials. 3 Biotech, 11(1), 10. https://doi.org/10.1007/s13205-020-02562-1
Ogawa, T., Misumi, M., & Sonoike, K. (2017). Estimation of photosynthesis in cyanobacteria by pulse-amplitude modulation chlorophyll fluorescence: Problems and solutions. Photosynthesis Research, 133, 63–73. https://doi.org/10.1007/s11120-017-0367-x
Tan, C. H., Show, P. L., Chang, J. S., Ling, T. C., & Lan, J. C. W. (2015). Novel approaches of producing bioenergies from microalgae: A recent review. Biotechnology Advances, 33(6), 1219–1227. https://doi.org/10.1016/j.biotechadv.2015.02.013
Sarsekeyeva, F., Zayadan, B. K., Usserbaeva, A., Bedbenov, V. S., Sinetova, M. A., & Los, D. A. (2015). Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynthesis Research, 125, 329–340. https://doi.org/10.1007/s11120-015-0103-3
Singh, S. P., Pathak, J., & Sinha, R. P. (2017). Cyanobacterial factories for the production of green energy and value-added products: An integrated approach for economic viability. Renewable and Sustainable Energy Reviews, 69, 578–595. https://doi.org/10.1016/j.rser.2016.11.110
Niyogi, K. K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annual Review of Plant Biology, 50(1), 333–359. https://doi.org/10.1146/annurev.arplant.50.1.333
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. Y. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology, 111(1), 1–61. https://doi.org/10.1099/00221287-111-1-1
Zavřel, T., Sinetova, M. A., & Červený, J. (2015). Measurement of chlorophyll a and carotenoids concentration in cyanobacteria. Bio-Protocol, 5(9), e1467–e1467. https://doi.org/10.21769/BioProtoc.1467
Schwarzkopf, M., Yoo, Y. C., Hückelhoven, R., Park, Y. M., & Proels, R. K. (2014). Cyanobacterial phytochrome2 regulates the heterotrophic metabolism and has a function in the heat and high-light stress response. Plant Physiology, 164(4), 2157–2166. https://doi.org/10.1104/pp.113.233270
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Singh, S. P., Rastogi, R. P., Sinha, R. P., & Häder, D.-P. (2013). Photosynthetic performance of Anabaena variabilis PCC 7937 under simulated solar radiation. Photosynthetica, 51(2), 259–266. https://doi.org/10.1007/s11099-013-0012-7
Kumar, V., Mondal, S., Gupta, A., Maurya, P. K., Sinha, R. P., Häder, D.-P., & Singh, S. P. (2021). Light-dependent impact of salinity on the ecophysiology of Synechococcus elongatus PCC 7942: Genetic and comparative protein structure analyses of UV-absorbing mycosporine-like amino acids (MAAs) biosynthesis. Environmental and Experimental Botany, 191, 104620. https://doi.org/10.1016/j.envexpbot.2021.104620
Genty, B., Briantais, J. M., & Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990(1), 87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
He, Y. Y., & Häder, D.-P. (2002). UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: protective effects of ascorbic acid and N-acetyl-L-cysteine. Journal of Photochemistry and Photobiology B: Biology., 66(2), 115–124. https://doi.org/10.1016/S1011-1344(02)00231-2
Rastogi, R. P., Singh, S. P., Häder, D.-P., & Sinha, R. P. (2010). Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochemical and Biophysical Research Communication, 397(3), 603–607. https://doi.org/10.1016/j.bbrc.2010.06.006
Rajneesh., Pathak, J., Chatterjee, A., Singh, S. P., & Sinha, R. P. (2017). Detection of reactive oxygen species (ROS) in cyanobacteria using the oxidant-sensing probe 2’, 7’-Dichlorodihydrofluorescein diacetate (DCFH-DA). Bio-protocol, 7(17), 2545–2545. https://doi.org/10.21769/BioProtoc.2545
Chen, Z., Tian, Y., Zhu, C., Liu, B., Zhang, Y., Lu, Z., Zhou, Q., & Wu, Z. (2018). Sensitive detection of oxidative DNA damage in cyanobacterial cells using supercoiling-sensitive quantitative PCR. Chemosphere, 211, 164–172. https://doi.org/10.1016/j.chemosphere.2018.06.154
Bethke, P. C., Lonsdale, J. E., Fath, A., & Jones, R. L. (1999). Hormonally regulated programmed cell death in barley aleurone cells. The Plant Cell, 11(6), 1033–1045. https://doi.org/10.1105/tpc.11.6.1033
Swapnil, P., Yadav, A. K., Srivastav, S., Sharma, N. K., Srikrishna, S., & Rai, A. K. (2017). Biphasic ROS accumulation and programmed cell death in a cyanobacterium exposed to salinity (NaCl and Na2SO4). Algal Research, 23, 88–95. https://doi.org/10.1016/j.algal.2017.01.014
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology, 59(2), 309–314. https://doi.org/10.1104/pp.59.2.309
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Steiger, S., Schäfer, L., & Sandmann, G. (1999). High-light-dependent upregulation of carotenoids and their antioxidative properties in the cyanobacterium Synechocystis PCC 6803. Journal Photochemistry and Photobiology B: Biology, 52(1–3), 14–18. https://doi.org/10.1016/S1011-1344(99)00094-9
Latifi, A., Ruiz, M., & Zhang, C. C. (2009). Oxidative stress in cyanobacteria. FEMS Microbiology Reviews, 33(2), 258–278. https://doi.org/10.1111/j.1574-6976.2008.00134.x
Hargreaves, A., Taiwo, F. A., Duggan, O., Kirk, S. H., & Ahmad, S. I. (2007). Near-ultraviolet photolysis of β-phenylpyruvic acid generates free radicals and results in DNA damage. Journal of Photochemistry and Photobiology B: Biology, 89(2–3), 110–116. https://doi.org/10.1016/j.jphotobiol.2007.09.007
Gao, Y., Xiong, W., Li, X. B., Gao, C. F., Zhang, Y. L., Li, H., & Wu, Q. Y. (2009). Identification of the proteomic changes in Synechocystis sp. PCC 6803 following prolonged UV-B irradiation. Journal of Experimental Botany, 60(4), 1141–1154. https://doi.org/10.1093/jxb/ern356
Rinalducci, S., Hideg, E., Vass, I., & Zolla, L. (2006). Effect of moderate UV-B irradiation on Synechocystis PCC 6803 biliproteins. Biochemical and Biophysical Research Communications, 341(4), 1105–1112. https://doi.org/10.1016/j.bbrc.2006.01.070
Kannaujiya, V. K., & Sinha, R. P. (2017). Impacts of diurnal variation of ultraviolet-B and photosynthetically active radiation on phycobiliproteins of the hot-spring cyanobacterium Nostoc sp. strain HKAR-2. Protoplasma, 254(1), 423–433. https://doi.org/10.1007/s00709-016-0964-0
Sinha, R. P., & Häder, D.-P. (2003). Biochemistry of phycobilisome disassembly by ultraviolet-B radiation in cyanobacteria. Recent Research Development in Biochemistry, 4, 945–955. https://doi.org/10.1016/j.jphotobiol.2014.09.020
Zakar, T., Laczko-Dobos, H., Toth, T. N., & Gombos, Z. (2016). Carotenoids assist in cyanobacterial photosystem II assembly and function. Frontier in Plant Science, 7, 295. https://doi.org/10.3389/fpls.2016.00295
Khanthasuwan, S., Incharoensakdi, A., & Jantaro, S. (2019). Response of Synechocystis sp. PCC 6803 to UV radiations by alteration of polyamines associated with thylakoid membrane proteins. World Journal of Microbiology and Biotechnology, 35, 1–9. https://doi.org/10.1007/s11274-018-2580-y
Rastogi, R. P., Singh, S. P., Incharoensakdi, A., Häder, D.-P., & Sinha, R. P. (2014). Ultraviolet radiation-induced generation of reactive oxygen species, DNA damage and induction of UV-absorbing compounds in the cyanobacterium Rivularia sp. HKAR-4. South African Journal of Botany, 90, 163–169. https://doi.org/10.1016/j.sajb.2013.11.006
Moon, Y. J., Kim, S. I., & Chung, Y. H. (2012). Sensing and responding to UV-A in cyanobacteria. International Journal of Molecular Sciences., 13(12), 16303–16332. https://doi.org/10.3390/ijms131216303
Schmitz, O., Katayama, M., Williams, S. B., Kondo, T., & Golden, S. S. (2000). CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science, 289(5480), 765–768. https://doi.org/10.1126/science.289.5480.765
Katayama, M., Kondo, T., Xiong, J., & Golden, S. S. (2003). ldpA encodes an iron-sulfur protein involved in light-dependent modulation of the circadian period in the cyanobacterium Synechococcus elongatus PCC 7942. Journal of Bacteriology, 185(4), 1415–1422. https://doi.org/10.1128/JB.185.4.1415-1422.2003
Acknowledgements
Prashant R. Singh (09/013(0795)/2018-EMR-I) is thankful to the Council of Scientific and Industrial Research, New Delhi, India for the financial support in the form of junior and senior research fellowships. We thank Dr. Vipin Kumar Singh for his valuable suggestions. The authors are thankful to the Head and Programme Coordinator (CAS), Dept. of Botany and Interdisciplinary School of Life Sciences (ISLS), Banaras Hindu University, Varanasi, India, for providing instrumentation facilities. Incentive grant received from IoE (Scheme No. 6031), Banaras Hindu University, Varanasi, India, to Rajeshwar P. Sinha is highly acknowledged.
Author information
Authors and Affiliations
Contributions
PRS: performed the experiments, analyzed the experimental results, statistical analysis and wrote the manuscript; JP: helped in writing the paper, data analysis and critically reviewed the manuscript; Rajneesh and HA: helped in the experimental work; DPH: suggested the research idea and reviewed the manuscript; RPS: designed the experiments, provided laboratory facilities, and reviewed the manuscript. All the authors accepted the final version of our manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, P.R., Pathak, J., Rajneesh et al. Physiological responses of the cyanobacterium Synechocystis sp. PCC 6803 under rhythmic light variations. Photochem Photobiol Sci 22, 2055–2069 (2023). https://doi.org/10.1007/s43630-023-00429-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00429-x