Abstract
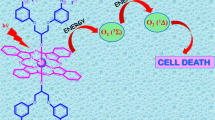
In this study, Schiff base substituted phthalocyanine complexes (Zn1c, Zn2c) and their quaternized derivatives (Q-Zn1c, Q-Zn2c) were synthesized for the first time. Their structures have been characterized by FT-IR, 1H-NMR, UV–Vis, mass spectrometry and elemental analysis as well as. The photophysicochemical properties (fluorescence, singlet oxygen and photodegradation quantum yield) of these novel complexes were investigated in dimethylsulfoxide (DMSO) for both non-ionic and quaternized cationic phthalocyanine complexes and in aqueous solution for quaternized cationic phthalocyanine complexes. Water soluble cationic phthalocyanine compounds gave good singlet oxygen quantum yield (0.65 for Q-Zn1c, 0.66 for Q-Zn2c in DMSO; 0.65 for Q-Zn2c in aqueous solution). The binding of Q-Zn1c and Q-Zn2c to BSA/DNA was studied by using UV–Vis and fluorescence spectroscopy and these. Studies indicate that the mechanism of BSA quenching by quaternized zinc(II) phthalocyanines was static quenching. Quaternized zinc(II) phthalocyanines interacted with ct-DNA by intercalation. Quaternized zinc(II) phthalocyanines caused a decrease in cell viability and triggered apoptotic cell death after PDT was applied at a concentration that did not have a toxic effect on their own. Q-Zn1c and Q-Zn2c mediated PDT reduced the activity of SOD, CAT, GSH while increased MDA level in the prostate cancer cells. Furthermore, expression of apoptotic proteins after PDT was examined. The results revealed that the synthesized water soluble quaternized zinc(II) phthalocyanine complexes (Q-Zn1c and Q-Zn2c) are promising potential photosensitizers for PDT.



















Similar content being viewed by others
Data availability
No data was used for the research described in the article.
References
Dai, J., Wu, X., Ding, S., Lou, X., Xia, F., Wang, S., & Hong, Y. (2020). Aggregation-induced emission photosensitizers: from molecular design to photodynamic therapy. Journal of Medicinal Chemistry, 63, 1996–2012. https://doi.org/10.1021/acs.jmedchem.9b02014
Nackiewicz, J., Kliber-Jasik, M., & Skonieczna, M. (2019). A novel pro-apoptotic role of zinc octacarboxyphthalocyanine in melanoma me45 cancer cell’s photodynamic therapy (PDT). Journal of Photochemistry and Photobiology, B: Biology, 190, 146–153. https://doi.org/10.1016/j.jphotobiol.2018.12.002
Aksel, M., Bozkurt-Girit, O., & Bilgin, M. D. (2020). Pheophorbide a-mediated sonodynamic, photodynamic and sonophotodynamic therapies against prostate cancer. Photodiagnosis and Photodynamic Therapy., 31, 101909. https://doi.org/10.1016/j.pdpdt.2020.101909
Yano, T., & Wang, K. K. (2020). Photodynamic therapy for gastrointestinal cancer. Photochemistry and Photobiology, 96, 517–523. https://doi.org/10.1111/php.13206
Matshitse, R., Tshiwawa, T., Managa, M., Nwaji, N., Lobb, K., & Nyokong, T. (2020). Theoretical and photodynamic therapy characteristics of heteroatom doped detonation nanodiamonds linked to asymmetrical phthalocyanine for eradication of breast cancer cells. Journal of Luminescence., 227, 117465. https://doi.org/10.1016/j.jlumin.2020.117465
Crous, A., Dhilip Kumar, S. S., & Abrahamse, H. (2019). Effect of dose responses of hydrophilic aluminium (III) phthalocyanine chloride tetrasulphonate based photosensitizer on lung cancer cells. Journal of Photochemistry and Photobiology B: Biology, 194, 96–106. https://doi.org/10.1016/j.jphotobiol.2019.03.018
Correia, J. H., Rodrigues, J. A., Pimenta, S., Dong, T., & Yang, Z. (2021). Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics, 13, 1332. https://doi.org/10.3390/pharmaceutics13091332
Jia, X., Yang, F. F., Li, J., Liu, J. Y., & Xue, J. P. (2013). Synthesis and in vitro photodynamic activity of oligomeric ethylene glycol-quinoline substituted zinc(II) phthalocyanine derivatives. Journal of Medicinal Chemistry, 56, 5797–5805. https://doi.org/10.1021/jm400722d
Hossain, A. M. S., Mendez-Arriaga, J. M., Xia, C., Xie, J., & Gomez-Ruiz, S. (2022). Metal complexes with ONS donor schiff bases. A review. Polyhedron, 217, 115692. https://doi.org/10.1016/j.poly.2022.115692
Yüzeroğlu, M., Karaoğlan, G. K., Köse, G. G., & Erdoğmuş, A. (2021). Synthesis of new zinc phthalocyanines including Schiff base and halogen; photophysical, photochemical, and fluorescence quenching studies. Journal of Molecular Structure, 1238, 130423. https://doi.org/10.1016/j.molstruc.2021.130423
Mi, Y., Chen, Y., Tan, W., Zhang, J., Li, Q., & Guo, Z. (2022). The influence of bioactive glyoxylate bearing Schiff base on antifungal and antioxidant activities to chitosan quaternary ammonium salts. Carbohydrate Polymers, 278, 118970. https://doi.org/10.1016/j.carbpol.2021.118970
Ahmed, Y. M., & Mohamed, G. G. (2021). Synthesis, spectral characterization, antimicrobial evaluation and molecular docking studies on new metal complexes of novel Schiff base derived from 4,6-dihydroxy-1,3-phenylenediethanone. Journal of Molecular Structure, 1256, 132496. https://doi.org/10.1016/j.molstruc.2022.132496
Sen, P., Yildiz, S. Z., Tuna, M., & Canlica, M. (2014). Preparation of aldehyde substituted phthalocyanines with improved yield and their use for Schiff base metal complex formation. Journal of Organometallic Chemistry, 769, 38–45. https://doi.org/10.1016/j.jorganchem.2014.07.007
Karaoğlan, G. K. (2022). Synthesis of new Schiff base and its Ni(II), Cu(II), Zn(II) and Co(II) complexes; photophysical, fluorescence quenching and thermal studies. Journal of Molecular Structure, 1256, 132534. https://doi.org/10.1016/j.molstruc.2022.132534
Çamur, M., Ahsen, V., & Durmuş, M. (2011). The first comparison of photophysical and photochemical properties of non-ionic, ionic and zwitterionic gallium (III) and indium (III) phthalocyanines. Journal of Photochemistry and Photobiology A: Chemistry, 219, 217–227. https://doi.org/10.1016/j.jphotochem.2011.02.014
Kutlu, Ö. D., Erdoğmuş, A., Şen, P., & Yıldız, S. Z. (2023). Peripherally tetra-Schiff base substituted metal-free and zinc(II) phthalocyanine, its water-soluble derivative: Synthesis, characterization, photo-physicochemical, aggregation properties and DNA/BSA binding activity. Journal of Molecular Structure. https://doi.org/10.1016/j.molstruc.2023.135375
Harmandar, K., Saglam, M. F., Sengul, I. F., Ekineker, G., Balcik-Ercin, P., Göksel, M., & Atilla, D. (2021). Novel triazole containing zinc(II)phthalocyanine Schiff bases: determination of photophysical and photochemical properties for photodynamic cancer therapy. Inorganica Chimica Acta, 519, 120286. https://doi.org/10.1016/j.ca.2021.120286
Ünlü, S., Elmalı, F. T., Atmaca, G. Y., & Erdoğmuş, A. (2022). Synthesis of axially Schiff base new substituted silicon phthalocyanines and investigation of photochemical and sono-photochemical properties. Photodiagnosis and Photodynamic Therapy, 40, 103192. https://doi.org/10.1016/j.pdpdt.2022.103192
Durmuş, M., Erdoğmuş, A., Ogunsipe, A., & Nyokong, T. (2009). The synthesis and photophysicochemical behaviour of novel water-soluble cationic indium(III) phthalocyanine. Dyes and Pigments, 82, 244–250. https://doi.org/10.1016/j.dyepig.2009.01.008
Wood, S. R., Holroyd, J. A., & Brown, S. B. (1997). The subcellular localization of Zn(II) phthalocyanines and their redistribution on exposure to light. Photochemistry and Photobiology, 65, 397–402. https://doi.org/10.1111/j.1751-1097.1997.tb08577.x
Idowu, M., Ogunsipe, A., & Nyokong, T. (2007). Excited state dynamics of zinc and aluminum phthalocyanine carboxylates. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 68, 995–999. https://doi.org/10.1016/j.saa.2007.01.025
Ilgün, C., Sevim, A. M., Çakar, S., Özacar, M., & Gül, A. (2021). Novel Co and Zn-phthalocyanine dyes with octa-carboxylic acid substituents for DSSCs. Solar Energy, 218, 169–179. https://doi.org/10.1016/j.solener.2021.02.042
Günsel, A., Atmaca, G. Y., Taslimi, P., Bilgiçli, A. T., Gülçin, İ, Erdoğmuş, A., & Yarasir, M. N. (2020). Synthesis, characterization, photo-physicochemical and biological properties of water-soluble tetra-substituted phthalocyanines: antidiabetic, anticancer and anticholinergic potentials. Journal of Photochemistry and Photobiology A: Chemistry, 396, 112511. https://doi.org/10.1016/j.jphotochem.2020.112511
Sharman, W. M., Kudrevich, S. V., & van Lier, J. E. (1996). Novel water-soluble phthalocyanines substituted with phosphonate moieties on the benzo rings. Tetrahedron Letters, 37, 5831–5834. https://doi.org/10.1016/0040-4039(96)01243-9
Zhang, L., Wang, A., Lu, S., Zhou, L., Zhou, J., Lin, Y., & Wei, S. (2015). The influences of the number of the ammonium groups and their arrangement manner on the photophysical properties of the quaternized zinc phthalocyanines. Inorganic Chemistry Communications, 53, 15–19. https://doi.org/10.1016/j.inoche.2015.01.009
Çakır, V., Göksel, M., Durmuş, M., & Bıyıklıoğlu, Z. (2016). Synthesis and photophysicochemical properties of novel water soluble phthalocyanines. Dyes and Pigments, 125, 414–425. https://doi.org/10.1016/j.dyepig.2015.10.035
Erdoğmuş, A., & Nyokong, T. (2010). Synthesis of zinc phthalocyanine derivatives with improved photophysicochemical properties in aqueous media. Journal of Molecular Structure, 977, 26–38. https://doi.org/10.1016/j.molstruc.2010.04.048
Yılmaz, H. E., Bağda, E., Bağda, E., & Durmuş, M. (2021). Interaction of water soluble cationic gallium(III) phthalocyanines with different G-quadruplex DNAs. Polyhedron, 208, 115404. https://doi.org/10.1016/j.poly.2021.115404
Sen, P., & Nyokong, T. (2021). Promising photodynamic antimicrobial activity of polyimine substituted zinc phthalocyanine and its polycationic derivative when conjugated to nitrogen, sulfur, co-doped graphene quantum dots against Staphylococcus aureus. Photodiagnosis and Photodynamic Therapy, 34, 102300. https://doi.org/10.1016/j.pdpdt.2021.102300
Kırbaç, E., & Erdoğmuş, A. (2020). New non-peripherally substituted zinc phthalocyanines; synthesis, and comparative photophysicochemical properties. Journal of Molecular Structure, 1202, 127392. https://doi.org/10.1016/j.molstruc.2019.127392
Atmaca, G. Y., Aksel, M., Keskin, B., Bilgin, M. D., & Erdoğmuş, A. (2021). The photo-physicochemical properties and in vitro sonophotodynamic therapy activity of Di-axially substituted silicon phthalocyanines on PC3 prostate cancer cell line. Dyes and Pigments, 184, 108760. https://doi.org/10.1016/j.dyepig.2020.108760
Sen, P., Sindelo, A., Mafukidze, D. M., & Nyokong, T. (2019). Synthesis and photophysicochemical properties of novel axially disubstituted silicon (IV) phthalocyanines and their photodynamic antimicrobial chemotherapy (PACT) activity against Staphylococcus aureus. Synthetic Metals, 258, 116203. https://doi.org/10.1016/j.synthmet.2019.116203
Günsel, A., Güzel, E., Bilgiçli, A. T., Atmaca, G. Y., Erdoğmuş, A., & Yaraşır, M. N. (2017). Synthesis and investigation of photophysicochemical properties of novel ketone-substituted gallium (III) and indium (III) phthalocyanines with high singlet oxygen yield for photodynamic therapy. Journal of Luminescence, 192, 888–892. https://doi.org/10.1016/j.jlumin.2017.08.014
Song, Y. M., Wu, Q., Yang, P. J., Luan, N. N., Wang, L. F., & Liu, Y. M. (2006). DNA Binding and cleavage activity of Ni(II) complex with all-trans retinoic acid. Journal of Inorganic Biochemistry, 100, 1685–1691. https://doi.org/10.1016/j.jinorgbio.2006.06.001
Kanchanadevi, S., Fronczek, F. R., David, C. I., Nandhakumar, R., & Mahalingam, V. (2021). Investigation of DNA/BSA binding and cytotoxic properties of new Co(II), Ni(II) and Cu(II) hydrazone complexes. Inorganica Chimica Acta, 526, 120536. https://doi.org/10.1016/j.ica.2021.120536
Baran, N. Y., & Saçak, M. (2018). Preparation of highly thermally stable and conductive Schiff base polymer: molecular weight monitoring and investigation of antimicrobial properties. Journal of Molecular Structure, 1163, 22–32. https://doi.org/10.1016/j.molstruc.2018.02.088
Hegazy, G. H., & Ali, H. I. (2012). Design, synthesis, biological evaluation, and comparative Cox1 and Cox2 docking of p-substituted benzylidenamino phenyl esters of ibuprofenic and mefenamic acids. Bioorganic and Medicinal Chemistry, 20, 1259–1270. https://doi.org/10.1016/j.bmc.2011.12.030
Bayrak, R., Albay, C., Koç, M., Altın, İ, Değirmencioğlu, İ, & Sökmen, M. (2016). Preparation of phthalocyanine/TiO2 nanocomposites for photocatalytic removal of toxic Cr(VI) ions. Process Safety and Environmental Protection, 102, 294–302. https://doi.org/10.1016/j.psep.2016.03.023
Dhupal, M., Oh, J. M., Tripathy, D. R., Kim, S. K., Koh, S. B., & Park, K. S. (2018). Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation. International Journal of Nanomedicine, 13, 6735–6750. https://doi.org/10.2147/IJN.S176087
Güzel, E., Atmaca, G. Y., Kuznetsov, A. E., Turkkol, A., Bilgin, M. D., & Erdoğmuş, A. (2022). Ultrasound versus light: Exploring photophysicochemical and sonochemical properties of phthalocyanine-based therapeutics, theoretical study, and in vitro evaluations. ACS Applied Bio Materials, 5, 1139–1150. https://doi.org/10.1021/acsabm.1c01199
Yalçin, A., Şarkici, G., & Kolaç, U. K. (2020). PKR inhibitors suppress endoplasmic reticulum stress and subdue glucolipotoxicity-mediated impairment of insulin secretion in pancreatic beta cells. Turkish Journal of Biology, 44, 93–102. https://doi.org/10.3906/biy-1909-20
Kolac, U. K., Eken, M. K., Ünübol, M., Yalcin, G. D., & Yalcin, A. (2021). The effect of gestational diabetes on the expression of mitochondrial fusion proteins in placental tissue. Placenta, 115, 106–114. https://doi.org/10.1016/j.placenta.2021.09.015
Çakır, V., Çakır, D., Göksel, M., Durmuş, M., Bıyıklıoğlu, Z., & Kantekin, H. (2015). Synthesis, photochemical, bovine serum albumin and DNA binding properties of tetrasubstituted zinc phthalocyanines and their water soluble derivatives. Journal of Photochemistry and Photobiology A: Chemistry, 299, 138–151. https://doi.org/10.1016/j.jphotochem.2014.11.007
Yanık, H., Al-Raqa, S. Y., Aljuhani, A., & Durmuş, M. (2016). The synthesis of novel directly conjugated zinc(II) phthalocyanine via palladium-catalyzed SuzukieMiyaura cross-coupling reaction and its quaternized water-soluble derivative: Investigation of photophysical and photochemical properties. Dyes and Pigments, 134, 531–540. https://doi.org/10.1016/j.dyepig.2016.08.009
Çolak, S., Durmuş, M., & Yıldız, S. Z. (2016). Tetrakis{2-[N-((3-morpholino)propyl)carbamate]oxyethyl} zinc(II) phthalocyanine and its water soluble derivatives: synthesis, photophysical, photochemical and protein binding properties. Journal of Photochemistry and Photobiology A: Chemistry, 325, 125–134. https://doi.org/10.1016/j.jphotochem.2016.04.006
Nyokong, T. (2007). Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coordination Chemistry Reviews, 251, 1707–1722. https://doi.org/10.1016/j.ccr.2006.11.011
Çakır, V., Çakır, D., Pişkin, M., Durmuş, M., & Bıyıklıoğlu, Z. (2014). Water soluble peripheral and non-peripheral tetrasubstituted zinc phthalocyanines: synthesis, photochemistry and bovine serum albumin binding behavior. Journal of Luminescence, 154, 274–284. https://doi.org/10.1016/j.jlumin.2014.04.030
Amitha, G. S., & Vasudevan, S. (2020). DNA/BSA binding studies of peripherally tetra substituted neutral azophenoxy zinc phthalocyanine. Polyhedron, 175, 114208. https://doi.org/10.1016/j.poly.2019.114208
Murov, S. L., Carmichael, I., & Hug, G. L. (1993). Handbook of photochemistry. Marcel Dekker.
Amitha, G. S., & Vasudevan, S. (2020). DNA binding and cleavage studies of novel Betti base substituted quaternary Cu(II) and Zn(II) phthalocyanines. Polyhedron, 190, 114773. https://doi.org/10.1016/j.poly.2020.114773
de Melo Gomes, L. C., de Oliveira Cunha, A. B., Peixoto, L. F. F., Zanon, R. G., Botelho, F. V., Silva, M. J. B., Pinto-Fochi, M. E., Goes, R. M., de Paoli, F., & Ribeiro, D. L. (2023). Photodynamic therapy reduces cell viability, migration and triggers necroptosis in prostate tumor cells. Photochemical & Photobiological Sciences. https://doi.org/10.1007/s43630-023-00382-9
Atmaca, G. Y., Aksel, M., Bilgin, M. D., & Erdoğmuş, A. (2023). Comparison of sonodynamic, photodynamic and sonophotodynamic therapy activity of fluorinated pyridine substituted silicon phthalocyanines on PC3 prostate cancer cell line. Photodiagnosis and Photodynamic Therapy, 42, 103339. https://doi.org/10.1016/j.pdpdt.2023.103339
Ayaz, F., Yetkin, D., Yüzer, A., Demircioğlu, K., & İnce, M. (2022). Non-canonical anti-cancer, anti-metastatic, anti-angiogenic and immunomodulatory PDT potentials of water soluble phthalocyanine derivatives with imidazole groups and their intracellular mechanism of action. Photodiagnosis and Photodynamic Therapy, 39, 103035. https://doi.org/10.1016/j.pdpdt.2022.103035
Gryko, M., Łukaszewicz-Zając, M., Guzińska-Ustymowicz, K., Kucharewicz, M., Mroczko, B., & Algirdas, U. (2023). The caspase-8 and procaspase-3 expression in gastric cancer and non-cancer mucosa in relation to clinico-morphological factors and some apoptosis-associated proteins. Advances in Medical Sciences, 68(1), 94–100. https://doi.org/10.1016/j.advms.2023.02.001
Wang, Q., Suo, Y., Wang, X., Wang, Y., Tian, X., Gao, Y., Liu, N., & Liu, R. (2021). Study on the mechanism of photodynamic therapy mediated by 5-aminoketovalerate in human ovarian cancer cell line. Lasers in Medical Science, 36, 1873–1881. https://doi.org/10.1007/s10103-020-03226-5
Cui, X. Y., Park, S. H., & Park, W. H. (2020). Auranofin inhibits the proliferation of lung cancer cells via necrosis and caspase-dependent apoptosis. Oncology Reports, 44, 2715–2724. https://doi.org/10.3892/or.2020.7818
Tan, P., Cai, H., Wei, Q., Tang, X., Zhang, Q., Kopytynski, M., Yang, J., Yi, Y., Zhang, H., Gong, Q., Gu, Z., Chen, R., & Luo, K. (2021). Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials, 277, 121061. https://doi.org/10.1016/j.biomaterials.2021.121061
Rutz, J., Maxeiner, S., Juengel, E., Bernd, A., Kippenberger, S., Zöller, N., Chun, F. K. H., & Blaheta, R. A. (2019). Growth and proliferation of renal cell carcinoma cells is blocked by low curcumin concentrations combined with visible light irradiation. International Journal of Molecular Sciences, 20(6), 1464. https://doi.org/10.3390/ijms20061464
Hu, J., Song, J., Tang, Z., Wei, S., Chen, L., & Zhou, R. (2021). Hypericin-mediated photodynamic therapy inhibits growth of colorectal cancer cells via inducing S phase cell cycle arrest and apoptosis. European Journal of Pharmacology, 900, 174071. https://doi.org/10.1016/j.ejphar.2021.174071
Zafari, J., Zadehmodarres, S., Jouni, F. J., Bagheri-Hosseinabadi, Z., Najjar, N., & Asnaashari, M. (2020). Investigation into the effect of photodynamic therapy and cisplatin on the cervical cancer cell line (A2780). Journal of Lasers in Medical Sciences, 11, S85–S91.
Erdogan, O., Abbak, M., Demirbolat, G. M., Birtekocak, F., Aksel, M., Pasa, S., & Cevik, O. (2019). Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE, 14, e0216496. https://doi.org/10.1371/journal.pone.0216496
Aksel, M., Kesmez, Ö., Yavaş, A., & Bilgin, M. D. (2021). Titaniumdioxide mediated sonophotodynamic therapy against prostate cancer. Journal of Photochemistry and Photobiology B: Biology, 225, 112333. https://doi.org/10.1016/j.jphotobiol.2021.112333
Lu, J., Mao, Y., Feng, S., Li, X., Gao, Y., Zhao, Q., & Wang, S. (2022). Biomimetic smart mesoporous carbon nanozyme as a dual-GSH depletion agent and O2 generator for enhanced photodynamic therapy. Acta Biomaterialia, 148, 310–322. https://doi.org/10.1016/j.actbio.2022.06.001
Zhuo, Z., Song, Z., Ma, Z., Zhang, Y., Xu, G., & Chen, G. (2019). Chlorophyllin e6-mediated photodynamic therapy inhibits proliferation and induces apoptosis in human bladder cancer cells. Oncology Reports, 41(4), 2181–2193. https://doi.org/10.3892/or.2019.7013
Dos Santos, A. F., de Almeida, D. R. Q., Terra, L. F., Wailemann, R. A., Gomes, V. M., Arini, G. S., Ravagnani, F. G., Baptista, M. S., & Labriola, L. (2020). Fluence rate determines PDT efficiency in breast cancer cells displaying different GSH levels. Photochemistry And Photobiology, 96(3), 658–667. https://doi.org/10.1111/php.13182
Acknowledgements
This study was supported by The Scientific & Technological Research Council of Turkey (TUBITAK, Project no: 219Z084). The authors would like to acknowledge that this paper is submitted in partial fulfillment of the requirements for PhD degree at Yildiz Technical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest in this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kocaağa, N., Türkkol, A., Bilgin, M.D. et al. The synthesis of novel water-soluble zinc (II) phthalocyanine based photosensitizers and exploring of photodynamic therapy activities on the PC3 cancer cell line. Photochem Photobiol Sci 22, 2037–2053 (2023). https://doi.org/10.1007/s43630-023-00428-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00428-y