Abstract
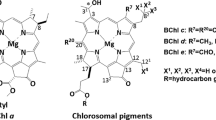
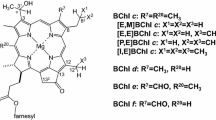
Bacteriochlorophyll (BChl) b has a unique π-conjugation system, in which the bacteriochlorin macrocycle is conjugated with the C8-ethylidene group. This π-system is converted easily to the chlorin macrocycle. However, the effects of the central magnesium in BChl b on this conversion are unclear. In this study, the isomerization kinetics of BChl b and its demetalated pigment, bacteriopheophytin (BPhe) b, was analyzed under weakly acidic conditions. BChl b exhibited faster acid-induced isomerization than BPhe b. These results were attributed to the stabilization of a cationic intermediate, whose C8-ethylidene group is protonated, during the isomerization of BChl b compared to BPhe b because of a difference in the electron densities of the π-conjugation systems between BChl b and BPhe b. High-performance liquid chromatography analyses indicated that BChl b was primarily isomerized to 3-acetyl Chl a, followed by demetalation. The reaction order was due to the slower demetalation kinetics of metallobacteriochlorins than metallochlorins. These results will be helpful for handling unstable BChl b and BPhe b. The reaction properties of BChl b and BPhe b demonstrated here will be helpful for understanding the in vivo formation of BPhe b, which acts as the primary electron acceptor in photosynthetic reaction center complexes in BChl b-containing purple photosynthetic bacteria.
Graphical abstract








Similar content being viewed by others
Abbreviations
- AcChl:
-
3-Acetyl chlorophyll
- AcPhe:
-
3-Acetyl pheophytin
- BChl:
-
Bacteriochlorophyll
- BPhe:
-
Bacteriopheophytin
- Chl:
-
Chlorophyll
- ESI:
-
Electrospray ionization
- HPLC:
-
High-performance liquid chromatography
- MS:
-
Mass spectrometry
- RC:
-
Reaction center
References
Scheer, H. (2006). An overview of chlorophylls and bacteriochlorophylls: Biochemistry, biophysics, functions and applications. In B. Grimm, R. J. Porra, W. Rüdiger, & H. Scheer (Eds.), Chlorophylls and bacteriochlorophylls: Biochemistry, biophysics, functions and applications (pp. 1–26). New York: Springer.
Tamiaki, H., & Kunieda, M. (2011). Photochemistry of chlorophylls and their analogs. In K. M. Kadish, K. M. Smith, & R. Guilard (Eds.), Handbook of Porphyrin Science (Vol. 11, pp. 223–290). World Scientific.
Croce, R., & van Amerongen, H. (2014). Natural strategies for photosynthetic light harvesting. Nature Chemical Biology, 10, 492–501.
Diers, J. R., Kirmaier, C., Taniguchi, M., Lindsey, J. S., Bocian, D. F., & Holten, D. (2021). A perspective on the redox properties of tetrapyrrole macrocycle. Physical Chemistry Chemical Physics, 23, 19130–19140.
Hoogewerf, G. J., Jung, D. O., & Madigan, M. T. (2003). Evidence for limited species diversity of bacteriochlorophyll b-containing purple nonsulfur anoxygenic phototrophs in freshwater habitats. FEBS Microbiology Letters, 218, 359–364.
Imhoff, J. F., Rahn, T., Künzel, S., & Neulinger, S. C. (2018). Photosynthesis is widely distributed among proteobacteria as demonstrated by the phylogeny of PufLM reaction center proteins. Frontiers in Microbiology, 8, 2679.
Kimura, Y., Yamashita, T., Seto, R., Imanishi, M., Honda, M., Nakagawa, S., Saga, Y., Takenaka, S., Yu, L.-J., Madigan, M. T., & Wang-Otomo, Z.-Y. (2021). Circular dichroism and resonance Raman spectroscopies of bacteriochlorophyll b-containing LH1-RC complexes. Photosynthesis Research, 148, 77–86.
Jay, F., Lambillotte, M., Stark, W., & Muhlethaler, K. (1984). The preparation and characterization of native photoreceptor units from the thylakoids of Rhodopseudomonas viridis. EMBO Journal, 3, 773–776.
Tsukatani, Y., Yamamoto, H., Harada, J., Yoshitomi, T., Nomata, J., Kasahara, M., Mizoguchi, T., Fujita, Y., & Tamiaki, H. (2013). An unexpectedly branched biosynthetic pathway for bacteriochlorophyll b capable of absorbing near-infrared light. Scientific Reports, 3, 1217.
Brockmann, H., Jr., & Lipinski, A. (1983). Bacteriochlorophyll g. A new bacteriochlorophyll from Heliobacterium chlorum. Archives of Microbiology, 136, 17–19.
Michalski, T. J., Hunt, J. E., Bowman, M. K., Smith, U., Bardeen, K., Gest, H., Norris, J. R., & Katz, J. J. (1987). Bacteriochlorophyll g: Properties and some speculations on a possible primary role for bacteriochlorophylls b and g in the biosynthesis of chlorophylls. Proceedings of the National Academy of Sciences of the United States of America, 84, 2570–2574.
Deisenhofer, J., Epp, O., Miki, K., Huber, R., & Michel, H. (1984). X-ray structure analysis of a membrane protein complex. Electron density map at 3 Å resolution and a model of the chromatophores of the photosynthetic reaction center from Rhodopseudomonas viridis. Journal of Molecular Biology, 180, 385–398.
Qian, P., Siebert, C. A., Wang, P., Canniffe, D. P., & Hunter, C. N. (2018). Cryo-EM structure of the Blastochloris viridis LH1-RC complex at 2.9 Å. Nature, 556, 203–208.
Beer-Romero, P., Favinger, J. L., & Gest, H. (1988). Distinctive properties of bacilliform photosynthetic heliobacteria. FEMS Microbiology Letters, 49, 451–454.
Kobayashi, M., Hamano, T., Akiyama, M., Watanabe, T., Inoue, K., Oh-oka, H., Amesz, J., Yamamura, M., & Kise, H. (1998). Light-independent isomerization of bacteriochlorophyll g to chlorophyll a catalyzed by weak acid in vitro. Analytica Chimica Acta, 365, 199–203.
Kobayashi, M., Yamamura, M., Akutsu, S., Miyake, J., Hara, M., Akiyama, M., & Kise, H. (1998). Successfully controlled isomerization and pheophytinization of bacteriochlorophyll b by weak acid in the dark in vitro. Analytica Chimica Acta, 361, 285–290.
Kolbasov, D., Srivatsan, N., Ponomarenko, N., Jäger, M., & Norris, J. R., Jr. (2003). Modeling charge transfer in oxidized bacterial antenna complexes. The Journal of Physical Chemistry B, 107, 2386–2393.
Kunieda, M., Mizoguchi, T., & Tamiaki, H. (2004). Synthesis and optical properties of stable 8-alkylidene-bacteriochlorins mimicking the molecular structures of natural bacteriochlorophylls-b and g. Tetrahedron, 60, 11349–11357.
Ferlez, B., Dong, W., Siavashi, R., Redding, K., Hou, H. J. M., Golbeck, J. H., & van der Est, A. (2015). The effect of bacteriochlorophyll g oxidation on energy and electron transfer in reaction centers from Heliobacterium modesticaldum. The Journal of Physical Chemistry B, 119, 13714–13725.
Saga, Y., Yamashita, M., Masaoka, Y., Hidaka, T., Imanishi, M., Kimura, Y., & Nagasawa, Y. (2021). Excitation energy transfer from bacteriochlorophyll b in the B800 site to B850 bacteriochlorophyll a in light-harvesting complex 2. The Journal of Physical Chemistry B, 125, 2009–2017.
Mizoguchi, T., Oh-oka, H., & Tamiaki, H. (2005). Determination of stereochemistry of bacteriochlorophyll gF and 81-hydroxy-chlorophyll aF from Heliobacterium modesticaldum. Photochemistry and Photobiology, 81, 666–673.
Saga, Y., Miura, R., Sadaoka, K., & Hirai, Y. (2011). Kinetic analysis of demetalation of synthetic zinc cyclic tetrapyrroles possessing an acetyl group at the 3-position: Effects of tetrapyrrole structures and peripheral substitution. The Journal of Physical Chemistry B, 115, 11757–11762.
Saga, Y., Hirota, K., Harada, J., & Tamiaki, H. (2015). In vitro enzymatic activities of bacteriochlorophyll a synthase derived from the green sulfur photosynthetic bacterium Chlorobaculum tepidum. Biochemistry, 54, 4998–5005.
Smith, J. R. L., & Calvin, M. (1966). Studies on the chemical and photochemical oxidation of bacteriochlorophyll. Journal of the American Chemical Society, 88, 4500–4506.
Saga, Y., & Miyagi, K. (2018). Characterization of 3-acetyl chlorophyll a and 3-acetyl protochlorophyll a accommodated in the B800 binding sites in photosynthetic light-harvesting complex 2 in the purple photosynthetic bacterium Rhodoblastus acidophilus. Photochemistry and Photobiology, 94, 698–704.
Oba, T., Masada, Y., & Tamiaki, H. (1997). Convenient preparation of pheophytin b from plant extract through the C7-reduced intermediate. Bulletin of the Chemical Society of Japan, 70, 1905–1909.
Hirai, Y., Kashimura, K., & Saga, Y. (2011). Demetalation kinetics of chlorophyll derivatives possessing different substituents at the 7-position under acidic conditions. Photochemistry and Photobiology, 87, 302–307.
Hynninen, P. H. (1991). Protonation–deprotonation equilibria in tetrapyrroles. Part 1. Protonation titrations of 132-(demethoxycarbonyl)pheophytin a in methanolic hydrochloric acid by electronic absorption spectroscopy. Journal of the Chemical Society, Perkin Transactions, 2, 669–678.
Noy, D., Fiedor, L., Hartwich, G., Scheer, H., & Scherz, A. (1998). Metal-substituted bacteriochlorophylls. 2. Changes in redox potentials and electronic transition energies are dominated by intramolecular electrostatic interactions. Journal of the American Chemical Society, 120, 3684–3693.
Kobayashi, M., Ohashi, S., Iwamoto, K., Shiraiwa, Y., Kato, Y., & Watanabe, T. (2007). Redox potential of chlorophyll d in vitro. Biochimica et Biophyica Acta, 1767, 596–602.
Kobayashi, M., Yamamura, M., Akiyama, M., Kise, H., Inoue, K., Hara, M., Wakao, N., Yahara, K., & Watanabe, T. (1998). Acid resistance of Zn-bacteriochlorophyll a from an acidophilic bacterium Acidiphilium rubrum. Analytical Sciences, 14, 1149–1152.
Funding
This work was partially supported by Grants-in-Aid for Scientific Research (C) (JP18KT0094 and JP21K06104) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takashima, Y., Saga, Y. Isomerization kinetics of bacteriochlorophyll b and bacteriopheophytin b under acidic conditions. Photochem Photobiol Sci 21, 1193–1199 (2022). https://doi.org/10.1007/s43630-022-00207-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00207-1