Abstract
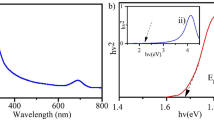
A novel solar light active photocatalyst, TiO2/kaolin-graphene carboxyl nanocomposite was synthesized by hydrothermal method for the degradation of cephalosporin antibiotic, cefuroxime sodium. The synthesized photocatalyst was characterized by various analytical and spectroscopic techniques, including Fourier transform infrared spectroscopy (FT-IR), powder X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX) thermogravimetry (TG), UV–Vis diffuse reflectance spectroscopy (DRS) and photoluminescence (PL). The prepared TiO2/kaolin-graphene carboxyl nanocomposite exhibited efficient photocatalytic degradation of methylene blue (MB) upon illumination with the solar simulator as compared to unmodified TiO2. The incorporation of both kaolin and graphene carboxyl was found to immobilize TiO2, enhancing the visible light absorption range of TiO2. Scavenger study revealed that hydroxyl radicals act as the main active species in the photocatalytic degradation process. The hydroxyl group present on kaolin surface reacts with photo-generated holes to increase the amount of hydroxyl radical, and further the graphene carboxyl plays a role to impede the recombination of photo-generated electron–hole pairs. Furthermore, the synthesized photocatalyst was found to degrade cefuroxime sodium within 90 min of sunlight illumination, indicating that TiO2/kaolin-graphene carboxyl nanocomposites would be very beneficial for pharmaceutical waste management through the advanced oxidation process. Mass spectral analysis was also carried out for elucidating the photocatalytic degradation pathway of cefuroxime sodium.
Graphical abstract
















Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Code availability
Not applicable.
References
Rathee, G., Singh, N., & Chandra, R. (2020). Simultaneous elimination of dyes and antibiotic with a hydrothermally generated NiAlTi layered double hydroxide adsorbent. ACS Omega, 5(5), 2368–2377. https://doi.org/10.1021/acsomega.9b03785
Jamee, R., & Siddique, R. (2019). Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. European Journal of Microbiology and Immunology, 9(4), 114–118. https://doi.org/10.1556/1886.2019.00018
Ribeiro, A. R., Sures, B., & Schmidt, T. C. (2018). Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environmental Pollution, 241, 1153–1166. https://doi.org/10.1016/j.envpol.2018.06.040
Kraemer, S. A., Ramachandran, A., & Perron, G. G. (2019). Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms, 7(6), 180. https://doi.org/10.3390/microorganisms7060180
Wozniak, T. J., & Hicks, J. R. (1991). Analytical profile of cefuroxime sodium. Analytical Profiles of Drug Substances, 20, 209–236. https://doi.org/10.1016/S0099-5428(08)60532-8
Anjum, M., Miandad, R., Waqas, M., Gehany, F., & Barakat, M. A. (2019). Remediation of wastewater using various nano-materials. Arabian Journal of Chemistry, 12(8), 4897–4919. https://doi.org/10.1016/j.arabjc.2016.10.004
Ferroudj, N., Nzimoto, J., Davidson, A., Talbot, D., Briot, E., Dupuis, V., Bée, A., Medjram, M. S., & Abramson, S. (2013). Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Applied Catalysis B: Environmental, 136, 9–18. https://doi.org/10.1016/j.apcatb.2013.01.046
Rasheed, T., Bilal, M., Nabeel, F., Adeel, M., & Iqbal, H. M. N. (2019). Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environment International, 122, 52–66. https://doi.org/10.1016/j.envint.2018.11.038
Yang, C., Li, Q., Tang, L., Bai, A., Song, H., & Yu, Y. (2016). Monodispersed colloidal zinc oxide nanospheres with various size scales: Synthesis, formation mechanism, and enhanced photocatalytic activity. Journal of Materials Science, 51(11), 5445–5459. https://doi.org/10.1007/s10853-016-9848-0
Irandost, M., Akbarzadeh, R., Pirsaheb, M., Asadi, A., Mohammadi, P., & Sillanpää, M. (2019). Fabrication of highly visible active N, S co-doped TiO2@ MoS2 heterojunction with synergistic effect for photocatalytic degradation of diclofenac: Mechanisms, modeling and degradation pathway. Journal of Molecular Liquids, 291, 111342. https://doi.org/10.1016/j.molliq.2019.111342
Pawar, M., Topcu Sendoğdular, S., & Gouma, P. (2018). A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. Journal of Nanomaterials, 2018, 5953609. https://doi.org/10.1155/2018/5953609
Khan, M., Yi, Z., Gul, S. R., Wang, Y., & Fawad, U. (2017). Visible-light-active silver-, vanadium-codoped TiO2 with improved photocatalytic activity. Journal of Materials Science, 52(10), 5634–5640. https://doi.org/10.1007/s10853-017-0798-y
Liao, C., Li, Y., & Tjong, S. C. (2020). Visible-light active titanium dioxide nanomaterials with bactericidal properties. Nanomaterials, 10(1), 124. https://doi.org/10.3390/nano10010124
Chong, M. N., Vimonses, V., Lei, S., Jin, B., Chow, C., & Saint, C. (2009). Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous and Mesoporous Materials, 117(1–2), 233–242. https://doi.org/10.1016/j.micromeso.2008.06.039
Wang, C., Shi, H., Zhang, P., & Li, Y. (2011). Synthesis and characterization of kaolinite/TiO2 nano-photocatalysts. Applied Clay Science, 53(4), 646–649. https://doi.org/10.1016/j.clay.2011.05.017
Li, X., Peng, K., Chen, H., & Wang, Z. (2018). TiO2 nanoparticles assembled on kaolinites with different morphologies for efficient photocatalytic performance. Scientific Reports, 8(1), 11663. https://doi.org/10.1038/s41598-018-29563-8
da Silva Lopes, J., Rodrigues, W. V., Oliveira, V. V., Braga, A. D. N. S., da Silva, R. T., França, A. A. C., da Paz, E. C., Osajima, J. A., & da Silva Filho, E. C. (2019). Modification of kaolinite from Pará/Brazil region applied in the anionic dye photocatalytic discoloration. Applied Clay Science, 168, 295–303. https://doi.org/10.1016/j.clay.2018.11.028
Štengl, V., Popelková, D., & Grygar, T. M. (2014). Composite pigments based on surface coated kaolin and metakaolin. Applied Clay Science, 101, 149–158. https://doi.org/10.1016/j.clay.2014.07.030
Li, X., & Tang, A. (2016). Pd modified kaolinite nanocomposite as a hydrogenation catalyst. RSC Advances, 6(19), 15585–15591. https://doi.org/10.1039/C5RA25387J
Henych, J., & Štengl, V. (2013). Feasible synthesis of TiO2 deposited on kaolin for photocatalytic applications. Clays and Clay Minerals, 61(3), 165–176. https://doi.org/10.1346/CCMN.2013.0610301
Adly, M. S., El-Dafrawy, S. M., & El-Hakam, S. A. (2019). Application of nanostructured graphene oxide/titanium dioxide composites for photocatalytic degradation of rhodamine B and acid green 25 dyes. Journal of Materials Research and Technology, 8(6), 5610–5622. https://doi.org/10.1016/j.jmrt.2019.09.029
Li, X., Yang, S., Sun, J., He, P., Xu, X., & Ding, G. (2014). Tungsten oxide nanowire-reduced graphene oxide aerogel for high-efficiency visible light photocatalysis. Carbon, 78, 38–48. https://doi.org/10.1016/j.carbon.2014.06.034
Rosu, M. C., Coros, M., Pogacean, F., Magerusan, L., Socaci, C., Turza, A., & Pruneanu, S. (2017). Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sciences, 70, 13–20. https://doi.org/10.1016/j.solidstatesciences.2017.05.013
Dreyer, D. R., Park, S., Bielawski, C. W., & Ruoff, R. S. (2010). The chemistry of graphene oxide. Chemical Society Reviews, 39(1), 228–240. https://doi.org/10.1039/b917103g
Krishnamoorthy, K., Veerapandian, M., Yun, K., & Kim, S. J. (2013). The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon, 53, 38–49. https://doi.org/10.1016/j.carbon.2012.10.013
Adán-Más, A., & Wei, D. (2013). Photoelectrochemical properties of graphene and its derivatives. Nanomaterials, 3(3), 325–356. https://doi.org/10.3390/nano3030325
Azira, N. M. N., Murizam, D., Halin, D. S. C., Idris, M. A., & Yunos, N. F. M. (2020). Preliminary study of hydrothermal synthesis of TiO2-GO composites as a high performance photocatalyst. IOP Conference Series: Materials Science and Engineering, 957(1), 012041. https://doi.org/10.1088/1757-899X/957/1/012041
Nguyen-Phan, T. D., Pham, V. H., Shin, E. W., Pham, H. D., Kim, S., Chung, J. S., Kim, E. J., & Hur, S. H. (2011). The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chemical Engineering Journal, 170(1), 226–232. https://doi.org/10.1016/j.cej.2011.03.060
Najafi, M., Kermanpur, A., Rahimipour, M. R., & Najafizadeh, A. (2017). Effect of TiO2 morphology on structure of TiO2-graphene oxide nanocomposite synthesized via a one-step hydrothermal method. Journal of Alloys and Compounds, 722, 272–277. https://doi.org/10.1016/j.jallcom.2017.06.001
Zhen, Q., Gao, L., Sun, C., Gong, H., Hu, P., Song, S., & Li, R. (2018). Honeycomb-like TiO2@ GO nanocomposites for the photodegradation of oxytetracycline. Materials Letters, 228, 318–321. https://doi.org/10.1016/j.matlet.2018.06.041
Khan, S. A., Arshad, Z., Shahid, S., Arshad, I., Rizwan, K., Sher, M., & Fatima, U. (2019). Synthesis of TiO2/Graphene oxide nanocomposites for their enhanced photocatalytic activity against methylene blue dye and ciprofloxacin. Composites Part B: Engineering, 175, 107120. https://doi.org/10.1016/j.compositesb.2019.107120
He, K., Chen, G., Zeng, G., Chen, A., Huang, Z., Shi, J., Peng, M., Huang, T., & Hu, L. (2018). Enhanced removal performance for methylene blue by kaolin with graphene oxide modification. Journal of the Taiwan Institute of Chemical Engineers, 89, 77–85. https://doi.org/10.1016/j.jtice.2018.04.013
Kutláková, K. M., Tokarský, J., Kovář, P., Vojtěšková, S., Kovářová, A., Smetana, B., Kukutschová, J., Čapková, P., & Matějka, V. (2011). Preparation and characterization of photoactive composite kaolinite/TiO2. Journal of Hazardous Materials, 188(1–3), 212–220. https://doi.org/10.1016/j.jhazmat.2011.01.106
Zhang, H., Wang, X., Li, N., Xia, J., Meng, Q., Ding, J., & Lu, J. (2018). Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Advances, 8(60), 34241–34251. https://doi.org/10.1039/C8RA06681G
Pan, N., Guan, D., Yang, Y., Huang, Z., Wang, R., Jin, Y., & Xia, C. (2014). A rapid low-temperature synthetic method leading to large-scale carboxyl graphene. Chemical Engineering Journal, 236, 471–479. https://doi.org/10.1016/j.cej.2013.10.060
Sun, W., Wang, C., Pan, W., Li, S., & Chen, B. (2017). Effects of natural minerals on the adsorption of 17β-estradiol and bisphenol A on graphene oxide and reduced graphene oxide. Environmental Science: Nano, 4(6), 1377–1388. https://doi.org/10.1039/C7EN00295E
Dodoo-Arhin, D., Buabeng, F. P., Mwabora, J. M., Amaniampong, P. N., Agbe, H., Nyankson, E., Obada, D. O., & Asiedu, N. Y. (2018). The effect of titanium dioxide synthesis technique and its photocatalytic degradation of organic dye pollutants. Heliyon, 4(7), e00681. https://doi.org/10.1016/j.heliyon.2018.e00681
Devi, R. S., Venckatesh, R., & Sivaraj, R. (2014). Synthesis of titanium dioxide nanoparticles by sol-gel technique. International Journal of Innovative Research in Science, Engineering and Technology, 3(8), 15206–15211. https://doi.org/10.15680/IJIRSET.2014.0308020
Mahalingam, T., Selvakumar, C., Kumar, E. R., & Venkatachalam, T. (2017). Structural, optical, morphological and thermal properties of TiO2-Al and TiO2-Al2O3 composite powders by ball milling. Physics Letters A, 381(21), 1815–1819. https://doi.org/10.1016/j.physleta.2017.02.053
Ramos, D. K. C., González, M. V., Muñóz, R. A. E., Cruz, J. S., De Moure-Flores, F. J., & Mayén-Hernández, S. A. (2020). Obtaining and characterization of TiO2-GO composites for photocatalytic applications. International Journal of Photoenergy, 2020, 3489218. https://doi.org/10.1155/2020/3489218
Ashkarran, A. A., Fakhari, M., Hamidinezhad, H., Haddadi, H., & Nourani, M. R. (2015). TiO2 nanoparticles immobilized on carbon nanotubes for enhanced visible-light photo-induced activity. Journal of Materials Research and Technology, 4(2), 126–132. https://doi.org/10.1016/j.jmrt.2014.10.005
Veer, D. H., Singh, R. M., & Kumar, H. (2017). Structural and optical characterization of ZnO-TiO2-SiO2 nanocomposites synthesized by sol-gel technique. Asian Journal of Chemistry, 29, 2391–2395. https://doi.org/10.14233/AJCHEM.2017.20690
Sun, Z., Li, C., Du, X., Zheng, S., & Wang, G. (2018). Facile synthesis of two clay minerals supported graphitic carbon nitride composites as highly efficient visible-light-driven photocatalysts. Journal of Colloid and Interface Science, 511, 268–276. https://doi.org/10.1016/j.jcis.2017.10.005
Salahudeen, N. (2018). Metakaolinization effect on the thermal and physiochemical propperties of kankara kaolin. Applied Science and Engineering Progress, 11(2), 127–135. https://doi.org/10.14416/j.ijast.2018.04.003
Li, J., Liu, D., Li, B., Wang, J., Han, S., Liu, L., & Wei, H. (2015). A bio-inspired nacre-like layered hybrid structure of calcium carbonate under the control of carboxyl graphene. CrystEngComm, 17(3), 520–525. https://doi.org/10.1039/C4CE01632G
Dubey, R. S., Krishnamurthy, K. V., & Singh, S. (2019). Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results in Physics, 14, 102390. https://doi.org/10.1016/j.rinp.2019.102390
Souza, A. E., Teixeira, S. R., Santos, G. T. A., & Longo, E. (2013). Addition of sedimentary rock to kaolinitic clays: Influence on sintering process. Cerâmica, 59, 147–155. https://doi.org/10.1590/S0366-69132013000100017
Zhang, L., Shi, T., Wu, S., & Zhou, H. (2014). Graphene/polystyrene nanocomposites synthesized via pickering emulsion polymerization. High Performance Polymers, 26(2), 156–165. https://doi.org/10.1177/0954008313504440
Kumar, S., Reddy, N. L., Kushwaha, H. S., Kumar, A., Shankar, M. V., Bhattacharyya, K., Halder, A., & Krishnan, V. (2017). Efficient electron transfer across ZnO-MoS2-RGO heterojunction for remarkably enhanced sunlight driven photocatalytic hydrogen evolution. Chemsuschem, 10, 3588–3603. https://doi.org/10.1002/cssc.201701024
Alamgir, K., Ahmad, S., Ahammed, N., & Naqvi, A. H. (2015). Thermal analysis and temperature dependent dielectric responses of Co doped anatase TiO2 nanoparticles. American Institute of Physics Conference Series, 1661(1), 080001. https://doi.org/10.1063/1.4915392
Dalod, A. R. M., Henriksen, L., Grande, T., & Einarsrud, M. A. (2017). Functionalized TiO2 nanoparticles by single-step hydrothermal synthesis: The role of the silane coupling agents. Beilstein Journal of Nanotechnology, 8(1), 304–312. https://doi.org/10.3762/bjnano.8.33
Sambaza, S. S., Maity, A., & Pillay, K. (2020). Polyaniline-coated TiO2 nanorods for photocatalytic degradation of bisphenol A in water. ACS Omega, 5(46), 29642–29656. https://doi.org/10.1021/acsomega.0c00628
Liu, H., Xie, S., Liao, J., Yan, T., Liu, Y., & Tang, X. (2018). Novel graphene oxide/bentonite composite for uranium (VI) adsorption from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry, 317(3), 1349–1360. https://doi.org/10.1007/s10967-018-5992-0
Liu, Y., Wang, L., Xue, N., Wang, P., Pei, M., & Guo, W. (2020). Ultra-highly efficient removal of methylene blue based on graphene oxide/TiO2/bentonite sponge. Materials, 13(4), 824. https://doi.org/10.3390/ma13040824
Mohammadi, R., & Sabourmoghaddam, N. (2020). TiO2-graphene/chitosan nanocomposite: preparation and its application for removal of anionic dyes. Asian Journal of Green Chemistry, 4(1), 11–32. https://doi.org/10.33945/SAMI/AJGC/2020.1.2
O’Regan, B., & Gratzel, M. (1991). A low-cost, high efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature, 353, 737–740. https://doi.org/10.1038/353737a0
Qaid, S. M. H., Al-Asbahi, B. A., Ghaithan, H. M., & Aldwayyan, A. S. (2021). Tuning the optical properties of MEH-PPV/PFO hybrid thin films via the incorporation of CsPbBr3 quantum dots. Coatings, 11, 154. https://doi.org/10.3390/coatings11020154
Karpovich, N. F., Pyachin, S. A., Pugachevskii, M. A., Burkov, A. A., Zaytsev, A. V., Makarevich, K. S., & Ri, E. K. (2015). Optical properties of anatase nanoparticles doped with tungsten. Journal of Applied Spectroscopy, 82(5), 767–772. https://doi.org/10.1007/s10812-015-0178-9
Nakamura, I., Negishi, N., Kutsuna, S., Ihara, T., Sugihara, S., & Takeuchi, K. (2000). Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. Journal of Molecular Catalysis A: Chemical, 161(1–2), 205–212. https://doi.org/10.1016/S1381-1169(00)00362-9
Rajender, G., Kumar, J., & Giri, P. K. (2018). Interfacial charge transfer in oxygen deficient TiO2-graphene quantum dot hybrid and its influence on the enhanced visible light photocatalysis. Applied Catalysis B: Environmental, 224, 960–972. https://doi.org/10.1016/j.apcatb.2017.11.042
Pal, M., Pal, U., Jiménez, J. M. G. Y., & Pérez-Rodríguez, F. (2012). Effects of crystallization and dopant concentration on the emission behavior of TiO2: Eu nanophosphors. Nanoscale Research Letters, 7(1), 1–12. https://doi.org/10.1186/1556-276X-7-1
Xiao, Q., Si, Z., Yu, Z., & Qiu, G. (2007). Sol-gel auto-combustion synthesis of samarium-doped TiO2 nanoparticles and their photocatalytic activity under visible light irradiation. Materials Science and Engineering: B, 137(1–3), 189–194. https://doi.org/10.1016/j.mseb.2006.11.011
Jing, L., Yuan, F., Hou, H., Xin, B., Cai, W., & Fu, H. (2005). Relationships of surface oxygen vacancies with photoluminescence and photocatalytic performance of ZnO nanoparticles. Science in China Series B: Chemistry, 48(1), 25–30. https://doi.org/10.1007/BF02990909
Mandal, S., Jain, N., Pandey, M. K., Sreejakumari, S. S., Shukla, P., Chanda, A., Som, S., Das, S., & Singh, J. (2019). Ultra-bright emission from Sr doped TiO2 nanoparticles through r-GO conjugation. Royal Society Open Science, 6(3), 190100. https://doi.org/10.1098/rsos.190100
Patil, A. B., Patil, K. R., & Pardeshi, S. K. (2010). Ecofriendly synthesis and solar photocatalytic activity of S-doped ZnO. Journal of Hazardous Materials, 183, 315–323. https://doi.org/10.1016/j.jhazmat.2010.07.026
Kang, Q., Cao, J., Zhang, Y., Liu, L., Xu, H., & Ye, J. (2013). Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. Journal of Materials Chemistry A, 1(18), 5766–5774. https://doi.org/10.1039/C3TA10689F
Cheng, Z., & Hu, X. (2017). Performance and degradation mechanism of a sequencing batch biofilm reactor combined with an electrochemical process for the removal of low concentrations of cefuroxime. Chemical Engineering Journal, 320, 93–103. https://doi.org/10.1016/j.cej.2017.03.037
Xiang, Q., Yu, J., & Wong, P. K. (2011). Quantitative characterization of hydroxyl radicals produced by various photocatalysts. Journal of Colloid and Interface Science, 357(1), 163–167. https://doi.org/10.1016/j.jcis.2011.01.093
Nosaka, Y., & Nosaka, A. Y. (2019). Comment on “Coumarin as a quantitative probe for hydroxyl radical formation in heterogeneous photocatalysis.” The Journal of Physical Chemistry C, 123(33), 20682–20684. https://doi.org/10.1021/acs.jpcc.9b04190
Acknowledgements
The authors wish to acknowledge The Management, Kuriakose Elias College for the support and encouragement throughout the study. The authors also wish to thank Sophisticated Analytical Instruments Facility (SAIF), STIC, Cochin for providing the necessary characterization facilities.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Rajan, M.S., Yoon, M. & Thomas, J. Kaolin-graphene carboxyl incorporated TiO2 as efficient visible light active photocatalyst for the degradation of cefuroxime sodium. Photochem Photobiol Sci 21, 509–528 (2022). https://doi.org/10.1007/s43630-022-00179-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00179-2