Abstract
Background
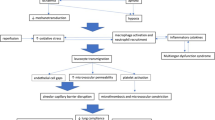
Tourniquet use is prevalent in the orthopaedic field to achieve a bloodless operating field, but it poses risks of local and systemic complications, including lung injury. This study aims to examine the effect of tourniquet application on the hindlimb of a rat to its lung.
Materials and Methods
This is an experimental study with 48 male Wistar strain rats as samples. The rats were divided into group A (n = 24), killed directly after fracturization and tourniquet application, and group B (n = 24), killed 14 days post-procedure. Each group was divided into four: group A1/B1 (control group, three hours tourniquet application without reperfusion interval), A2/B2 (5-min reperfusion between 2-h and 1-h tourniquet application), A3/B3 (10-min reperfusion), and A4/B4 (15-min reperfusion). The lung tissue was examined histologically within ten high-power fields (400 × magnification). The severity of lung injury was measured using the Lung Injury Score (LIS). The oxidative damage was measured by determining the malondialdehyde (MDA) level, using the TBARS (thiobarbituric acid reactive substance assay) method.
Results
There was a dose-dependent decrease of LIS and MDA in groups A and B with increasing reperfusion interval. Fifteen-minute reperfusion interval caused a 54.55% and 45.33% LIS reduction in groups A and B, respectively. All pair-wise group comparisons (p < 0.05) showed significant differences. Five-minute interval reduced the MDA level by 16.56% and 30.13% in groups A and B, respectively. All possible pair-wise comparisons in both groups A and B also showed a significant difference (p < 0.05).
Conclusions
Reperfusion interval is a possible clinical approach to mitigate the remote organ damage induced by limb ischemia–reperfusion injury.


Similar content being viewed by others
References
Kumar, K., Railton, C., & Tawfic, Q. (2016). Tourniquet application during anesthesia: “What we need to know?”. Journal of Anaesthesiology Clinical Pharmacology, 32(4), 424–430. https://doi.org/10.4103/0970-9185.168174.
Sharma, J. P., & Salhotra, R. (2012). Tourniquets in orthopedic surgery. Indian Journal of Orthopaedics, 46(4), 377–383. https://doi.org/10.4103/0019-5413.98824.
Odinsson, A., & Finsen, V. (2006). Tourniquet use and its complications in Norway. Journal of Bone and Joint Surgery British volume, 88-B(8), 1090–1092. https://doi.org/10.1302/0301-620X.88B8.17668.
Van der Spuy, L. A. (2012). Complications of the arterial tourniquet. Southern African Journal of Anaesthesia and Analgesia, 18(1), 14–18.
Leurcharusmee, P., Sawaddiruk, P., Punjasawadwong, Y., Chattipakorn, N., & Chattipakorn, S. C. (2018). The possible pathophysiological outcomes and mechanisms of tourniquet-induced ischemia-reperfusion injury during total knee arthroplasty. Oxidative Medicine and Cellular Longevity, 2018, 8087598. https://doi.org/10.1155/2018/8087598.
Chen, L., Zhao, H., Alam, A., et al. (2019). Postoperative remote lung injury and its impact on surgical outcome. BMC Anesthesiology, 19(1), 1–10. https://doi.org/10.1186/s12871-019-0698-6.
Lin, L., Wang, L., Bai, Y., et al. (2010). Pulmonary gas exchange impairment following tourniquet deflation: A prospective, single-blind clinical trial. Orthopedics, 33(6), 395. https://doi.org/10.3928/01477447-20100429-15.
Jin, Z., Suen, K. C., & Ma, D. (2016). Perioperative, “remote” acute lung injury: Recent update. Journal of Biomedical Research, 2016(31), 197–212. https://doi.org/10.7555/jbr.31.20160053.
Halladin, N. L., Zahle, F. V., Rosenberg, J., & Gögenur, I. (2014). Interventions to reduce tourniquet-related ischaemic damage in orthopaedic surgery: A qualitative systematic review of randomised trials. Anaesthesia, 69(9), 1033–1050. https://doi.org/10.1111/anae.12664.
Horlocker, T. T., Hebl, J. R., Gali, B., et al. (2006). Anesthetic, patient, and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesthesia and Analgesia, 102(3), 950–955. https://doi.org/10.1213/01.ane.0000194875.05587.7e.
Drysch, M., Wallner, C., Schmidt, S. V., et al. (2019). An optimized low-pressure tourniquet murine hind limb ischemia reperfusion model: Inducing acute ischemia reperfusion injury in C57BL/6 wild type mice. PLoS ONE, 14(1), e0210961. https://doi.org/10.1371/journal.pone.0210961.
Tran, T. P., Tu, H., Pipinos, I. I., Muelleman, R. L., Albadawi, H., & Li, Y.-L. (2011). Tourniquet-induced acute ischemia-reperfusion injury in mouse skeletal muscles: Involvement of superoxide. European Journal of Pharmacology, 650(1), 328–334. https://doi.org/10.1016/j.ejphar.2010.10.037.
Prodinger, P. M., Bürklein, D., Foehr, P., et al. (2018). Improving results in rat fracture models: Enhancing the efficacy of biomechanical testing by a modification of the experimental setup. BMC Musculoskeletal Disorders, 19(1), 1–8. https://doi.org/10.1186/s12891-018-2155-y.
Moreno, L. D., Waldman, S. D., & Grynpas, M. D. (2006). Sex differences in long bone fatigue using a rat model. Journal of Orthopaedic Research, 24(10), 1926–1932. https://doi.org/10.1002/jor.20250.
Hiltunen, A., Vuorio, E., & Aro, H. T. (1993). A standardized experimental fracture in the mouse tibia. Journal of Orthopaedic Research, 11(2), 305–312. https://doi.org/10.1002/jor.1100110219.
Otto, T. E., Patka, P., & Haarman, H. J. T. M. (1995). Closed fracture healing: A rat model. European Surgical Research, 27(4), 277–284. https://doi.org/10.1159/000129410.
Handool, K. O., Ibrahim, S. M., Kaka, U., et al. (2018). Optimization of a closed rat tibial fracture model. Journal of Experimental Orthopaedics. https://doi.org/10.1186/s40634-018-0128-6.
Crawford, R. S., Hashmi, F. F., Jones, J. E., et al. (2007). A novel model of acute murine hindlimb ischemia. Am J Physiol - Hear Circ Physiol, 292(2), 830–837. https://doi.org/10.1152/ajpheart.00581.2006.
Matute-Bello, G., Downey, G., Moore, B. B., et al. (2011). An official American thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. American Journal of Respiratory Cell and Molecular Biology, 44(5), 725–738. https://doi.org/10.1165/rcmb.2009-0210ST.
Mansour, Z., Charles, A. L., Kindo, M., et al. (2014). Remote effects of lower limb ischemia-reperfusion: Impaired lung, unchanged liver, and stimulated kidney oxidative capacities. BioMed Research International, 2014, 1–7. https://doi.org/10.1155/2014/392390.
Men, X., Han, S., Gao, J., et al. (2010). Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis. Acta Orthopaedica, 81(2), 263–267. https://doi.org/10.3109/17453671003587085.
Sotoudeh, A., Takhtfooladi, M. A., Jahanshahi, A., Asl, A. H. K., Takhtfooladi, A., & Khansari, M. (2012). Effect of N-acetylcysteine on lung injury induced by skeletal muscle ischemia-reperfusion: Histopathological study in rat model. Estudo Histopatólogi, 27(2), 168–171.
Wang, L., Chen, B., Lin, B., et al. (2018). Methylene blue attenuates lung injury induced by hindlimb ischemia reperfusion in rats. Mediators of Inflammation. https://doi.org/10.1155/2018/2508620.
Takhtfooladi, M. A., Jahanshahi, A., Sotoudeh, A., Jahanshahi, G., Takhtfooladi, H. A., & Aslani, K. (2013). Effect of tramadol on lung injury induced by skeletal muscle ischemia-reperfusion: An experimental study. Jornal Brasileiro de Pneumologia, 39(4), 434–439. https://doi.org/10.1590/S1806-37132013000400006.
Takhtfooladi, H., Takhtfooladi, M., Moayer, F., & Mobarakeh, S. (2015). Melatonin attenuates lung injury in a hind limb ischemia–reperfusion rat model. Revista Portuguesa de Pneumologia (English Edition), 21(1), 30–35. https://doi.org/10.1016/j.rppnen.2014.01.010.
Takhtfooladi, H. A., & Takhtfooladi, M. A. (2019). Effect of curcumin on lung injury induced by skeletal muscle ischemia/reperfusion in rats. Ulusal Travma ve Acil Cerrahi Dergisi, 25(1), 7–11. https://doi.org/10.5505/tjtes.2018.83616.
Hausenloy, D. J., & Yellon, D. M. (2009). Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis, 204(2), 334–341. https://doi.org/10.1016/j.atherosclerosis.2008.10.029.
Harkin, D. W., Barros D’Sa, A. A. B., McCallion, K., Hoper, M., & Campbell, F. C. (2002). Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. Journal of Vascular Surgery, 35(6), 1264–1273.
Jamshidi, F., Entezari, S., Alimian, M., Siamdoust, A., & Koleini, Z. S. (2016). Remote ischemic preconditioning in lower limb surgery; The hemodynamic and respiratory effects. Journl of Cellular and Molecular Anesthesia, 1(3), 97–102.
Song, S. Q., Gan, H. L., Zhang, J. Q., Feng, L., Sun, J. C., & Wang, S. X. (2015). Post-conditioning through lower limb ischemia-reperfusion can alleviate lung ischemia-reperfusion injury. International Journal of Clinical and Experimental Medicine, 8(9), 14953–14961.
Ferrari, R. S., & Andrade, C. F. (2015). Oxidative stress and lung ischemia-reperfusion injury. Oxidative Medicine and Cellular Longevity, 2015, 590987. https://doi.org/10.1155/2015/590987.
Li, C., Xu, M., Wu, Y., Li, Y.-S., Huang, W.-Q., & Liu, K.-X. (2014). Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: A randomized controlled study. Anesthesiology, 121(2), 249–259. https://doi.org/10.1097/ALN.0000000000000266.
Kalogeris, T., Baines, C. P., Krenz, M., & Korthuis, R. J. (2017). Ischemia/reperfusion. Comprehensive Physiology, 7(1), 113–170. https://doi.org/10.1002/cphy.c160006.
Peng, T. C., Jan, W. C., Tsai, P. S., & Huang, C. J. (2011). Heme oxygenase-1 mediates the protective effects of ischemic preconditioning on mitigating lung injury induced by lower limb ischemia-reperfusion in rats. Journal of Surgical Research, 167(2), e245–e253. https://doi.org/10.1016/j.jss.2010.06.010.
Kao, M. C., Jan, W. C., Tsai, P. S., Wang, T. Y., & Huang, C. J. (2011). Magnesium sulfate mitigates lung injury induced by bilateral lower limb ischemia-reperfusion in rats. Journal of Surgical Research, 171(1), e97–e106. https://doi.org/10.1016/j.jss.2011.03.028.
Hsu, K. Y., Tsai, P. S., Lee, J. J., Wang, T. Y., & Huang, C. J. (2011). Platonin mitigates acute lung injury induced by bilateral lower limb ischemia-reperfusion in rats. Journal of Surgical Research, 167(2), e255–e262. https://doi.org/10.1016/j.jss.2010.03.075.
Schofield, Z. V., Woodruff, T. M., Halai, R., Wu, M. C.-L., & Cooper, M. A. (2013). Neutrophils—a key component of ischemia-reperfusion injury. Shock, 40(6), 463–470. https://doi.org/10.1097/SHK.0000000000000044.
Moldoveanu, B., Otmishi, P., Jani, P., Walker, J., Sarmiento, X., Guardiola, J., et al. (2009). Inflammatory mechanisms in the lung. Journal of Inflammation Research, 2, 1–11.
Wu, Q., Zhong, Z.-M., Pan, Y., et al. (2015). Advanced oxidation protein products as a novel marker of oxidative stress in postmenopausal osteoporosis. Medical Science Monitor, 21, 2428–2432. https://doi.org/10.12659/MSM.894347.
Ibrahim, M. A. A., Elwan, W. M., & Elgendy, H. A. (2019). Role of scutellarin in ameliorating lung injury in a rat model of bilateral hind limb ischemia-reperfusion. Anatomical Record (Hoboken). https://doi.org/10.1002/ar.24175.
Demling, R., & LaLonde, C. (1989). Relationship between lung injury and lung lipid peroxidation caused by recurrent endotoxemia. The American Review of Respiratory Disease, 139(5), 1118–1124. https://doi.org/10.1164/ajrccm/139.5.1118.
Bajpai, J., Prakash, V., Kant, S., et al. (2017). Study of oxidative stress biomarkers in chronic obstructive pulmonary disease and their correlation with disease severity in north Indian population cohort. Lung India, 34(4), 324. https://doi.org/10.4103/lungindia.lungindia_205_16.
Budic, I., Pavlovic, D., Kitic, D., et al. (2013). Tourniquet-induced ischemia-reperfusion injuries during extremity surgery at children’s age: Impact of anesthetic chemical structure. Redox Report, 18(1), 20–26. https://doi.org/10.1179/1351000212y.0000000037.
Funding
This study was solely funded by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
All institutional and national guidelines for the care and use of laboratory animals were followed. This study has been reviewed and approved by the authors' Institutional Review Board.
Informed consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huwae, T.E.C.J., Santoso, A.R.B., Kesuma, W. et al. Reperfusion Interval as a Prevention of Lung Injury Due to Limb Ischemia–Reperfusion After Application of Tourniquet in Murine Experimental Study. JOIO 54, 704–710 (2020). https://doi.org/10.1007/s43465-020-00100-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-020-00100-y