Abstract
Background
The guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which was established in 1990 to streamline and standardize drug-approval review standards across Japan, the United States, and Europe. The ICH guidelines were established approximately 30 years ago, and, since then, temperatures have risen owing to global warming. Therefore, I verified whether the ICH guidelines correspond to the latest climate by using the Arrhenius equation, which is the basis for the ICH guidelines.
Methods
This study used an excerpt of the test conditions described in the ICH guidelines to calculate the mean kinetic temperature (MKT) for major Japanese cities based on temperature data from 1991 to 2020 (measured by the Japan Meteorological Agency).
Results
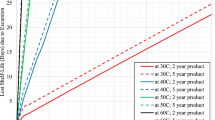
The study confirmed that the temperature conditions prescribed by the ICH guidelines for long-term storage tests were satisfied (see Fig. 1). Additionally, as drugs can be exposed to temperatures outside the specified range during distribution from the manufacturer to the final customer, data logs/loggers were utilized to calculate the MKT using the temperature history during transportation and storage.

Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Haynes JD. Worldwide virtual temperatures for product stability testing. J Pharm Sci. 1971;60:927–9.
Japan Pharmaceutical Manufacturers Association, Drug Evaluation Committee, Statistics / DM Subcommittee. Evaluation of stability test data of drugs containing new active ingredients – Study design and statistical estimation method for establishing re-test period and validity period, Japan Pharmaceutical Publication Center; 2005.
Materials for Drug Manufacturing Supervisor Workshop (FY1999). Ministry of Health and Welfare, Pharmaceutical and Medical Safety Bureau, sponsored by Federation of Pharmaceutical Manufacturers’ Associations of Japan; 1999, P. 10.
Pharmaceuticals and Medical Devices Agency, international council for harmonisation of technical requirements for pharmaceuticals for human use (ICH). https://www.pmda.go.jp/int-activities/int-harmony/ich/0014.html (Accessed 25 January 2020).
ICH. Welcome to the ICH Official Website. https://www.ich.org/ (Accessed 18 Feb 2020).
Tobias Kuners of Koenders, Is It Time to Stop Using Mean Kinetic Temperature (MKT) in Pharma Storage & Transport?; 2020. bioprocessonline.com.
Cabinet Order to determine the measurement units in article 3 of the supplementary provisions of the measurement act (Cabinet Order No. 358 of 1992) appended Table 3.
Katori N, Medical Device Regulatory Science Comprehensive Research Project, Research on systematization and international harmonization of drug manufacturing and quality control methods in the drug quality system, FY2012 Summary and Shared Research Report; 2013.
ICH harmonised tripartite guideline, stability testing of new drug substances and products; Q1a:(R2). https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (Accessed 25 Feb 2020).
Pharmaceuticals and Medical Devices Agency. Stability test guidelines. https://www.pmda.go.jp/files/000238766.pdf(2003) (Accessed 2 March 2020).
Seevers RH, Hofer J, Harber P, Ulrich DA, Bishara R. The use of Mean Kinetic Temperature (MKT) in the handling, storage, and distribution of temperature sensitive pharmaceuticals. Pharm Outsorcing. 2009;10:12–7.
Acknowledgements
I would like to thank Editage (www.editage.com) for English language editing.
Funding
This work did not receive any financial support.
Author information
Authors and Affiliations
Contributions
The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miura, E. Use of Mean Kinetic Temperature for Pharmaceuticals in Japan and Stability Monitoring in the 21st Century. Ther Innov Regul Sci 58, 184–191 (2024). https://doi.org/10.1007/s43441-023-00584-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00584-4