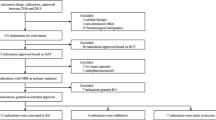
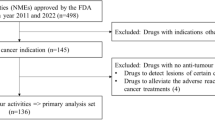
Abstract
The accelerated approval (AA) program in the USA has succeeded in expediting the regulatory approval of new cancer drugs based on surrogate endpoint data. It is unclear whether the AA program promotes overall drug development, including verification of the clinical benefit, as the verification of drugs granted AA often takes long time. To determine the impact of the AA program on overall drug development, the time required for verification of clinical benefits was compared between anticancer drugs that initially received AA and those that received regular approval (RA). It was found that anticancer drugs that were approved under the AA program took longer time for verification, suggesting that the program may delay the start of a confirmatory study, and there may be room for speeding up the process. In addition, discordance was found in the pivotal study between the USA and the EU and the USA and Japan for obtaining the indication for which AA was granted in the USA and a delay in the start of the confirmatory study for the AA indication was considered to lead to a delay in approval in the EU and Japan. Early initiation of confirmatory studies for AA indications is recommended to reduce the time that patients receive drugs with unproven benefit in the USA, as well as to deliver innovative new drugs to patients earlier in the EU and Japan.



Similar content being viewed by others
References
Beaver JA, Howie LJ, Pelosof L, et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–56.
Yamashita K, Kaneko M, Narukawa M. Regulatory characteristics and pivotal study design of US Food and Drug Administration approval of drugs for major vs. minor cancer. Eur J Clin Pharmacol. 2019;75(9):1193–1200.
Chen EY, Joshi SK, Tran A, et al. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern Med. 2019;179(5):642–7.
Fashoyin-Aje LA, Mehta GU, Beaver JA, et al. The on- and off-ramps of oncology accelerated approval. N Engl J Med. 2022;387(16):1439–42.
U.S. Department of Health and Human Services. Delays in Confirmatory Trials for Drug Applications Granted FDA's Accelerated Approval Raise Concerns https://oig.hhs.gov/oei/reports/OEI-01-21-00401.asp Published 29 September 2022. Accessed 20 December 2022.
U.S. Food and Drug Administration. Project FrontRunner https://www.fda.gov/about-fda/oncology-center-excellence/project-frontrunner Accessed 20 December 2022.
Friends of Cancer Research. Accelerating Investigation of New Therapies in Earlier Metastatic Treatment Settings Friends of Cancer Research Annual Meeting 2022 https://friendsofcancerresearch.org/wp-content/uploads/Accelerating_Investigation_Therapies_Earlier_Metastatic_Treatment_Settings.pdf Accessed 20 December 2022.
Cherla A, Naci H, Kesselheim AS, et al. Assessment of coverage in England of cancer drugs qualifying for US food and drug administration accelerated approval. JAMA Intern Med. 2021;181(4):490–8.
Nagasawa T, Aruga A. Potential impact of accelerated approval on the drug lags for anticancer drugs between the United States and Japan. Journal of Regulatory Science. 2020;2020(8):1–9.
U.S. Food and Drug Administration. Clinical Trial Considerations to Support Accelerated Approval of Oncology Therapeutics https://www.fda.gov/media/166431/download Published Mar 2023. Accessed 23 June 2023
U.S. Food and Drug Administration. Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics https://www.fda.gov/media/86377/download Published May 2014. Accessed 20 December 2022.
Beaver JA, Pazdur R. “Dangling” accelerated approvals in oncology. N Engl J Med. 2021;384(18): e68.
Nagai S. Flexible and expedited regulatory review processes for innovative medicines and regenerative medical products in the US, the EU, and Japan. Int J Mol Sci. 2019;20(15):3801.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Author information
Authors and Affiliations
Contributions
IA wrote the manuscript. IA and NM designed the study. IA performed the study. IA and NM analyzed the data.
Corresponding author
Ethics declarations
Conflict of Interest
Akira Ito is an employee of Daiichi Sankyo Co., Ltd., Tokyo, Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ito, A., Narukawa, M. Impact of the US Accelerated Approval for New Anticancer Drugs on Time to Verification of Benefit and Regulatory Approval in the EU and Japan. Ther Innov Regul Sci 58, 136–142 (2024). https://doi.org/10.1007/s43441-023-00577-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00577-3